J Adv Periodontol Implant Dent. 2025;17(3):145-151.
doi: 10.34172/japid.025.3895
Research Article
Effect of nonsurgical periodontal therapy on the eradication of gastric Helicobacter pylori infection: A randomized clinical trial
Parichehr Behfarnia Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, 1, * 
Reza Birang Data curation, Investigation, Project administration, Resources, Validation, Visualization, 1 
Shirin Rostami Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, 2 
Vahid Sebghatollahi Data curation, Investigation, Project administration, Supervision, Visualization, 3 
Author information:
1Department of Periodontics, Dental Implant Research Center, Dental Research Institute, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Periodontics, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
3Department of Internal Medicine, School of Medicine, AL-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
Abstract
Background.
This study aimed to assess the effectiveness of "a combined systemic and nonsurgical periodontal treatment" in eradicating gastric Helicobacter pylori in patients having the bacterium within their subgingival biofilm.
Methods.
This randomized clinical trial (RCT) investigated 102 patients diagnosed with peptic ulcer or dyspepsia and a positive stomach test for H. pylori infection (G+). Participants with a negative test for oral infection received only triple therapy (G3, n=38), and those positive for oral infection were randomly allocated to one of the two treatment regimens: a 14-day course of triple therapy (comprising antibiotics, antimicrobials, and proton pump inhibitors) alongside periodontal therapy (G1, n=32) or triple therapy alone (G2, n=32). The effectiveness of H. pylori eradication was assessed four weeks after treatment using the H. pylori stool antigen (stool Ag) test. Data analysis was performed using SPSS 22.
Results.
In the G2 and G3 groups, triple therapy achieved success rates of 52% and 84%, respectively. When periodontal therapy was integrated with triple therapy in the G1 group, the success rate was 80%. Significant differences were observed between the G1 and G2 groups (P=0.037) and also between the G3 and G2 groups (P=0.015). Conversely, no significant difference was found between the G1 and G3 groups (P>0.05).
Conclusion.
Periodontal therapy has the potential to substantially increase the efficacy of H. pylori eradication regimens for gastric infections.
Keywords: Helicobacter pylori, Nonsurgical periodontal debridement, Peptic ulcer, Scaling and root planing, Therapy
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This research study received financial support from the Vicechancellor for Research at Isfahan University of Medical Sciences (grant No.: 396174).
Introduction
Helicobacter pylori, a bacterium that inhabits the gastric mucosa, is a significant human pathogen. Its presence is strongly linked to the development of chronic gastritis, peptic ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma.1 Eradicating H. pylori promotes gastric ulcer healing and diminishes both the recurrence of ulcers and the incidence of associated gastrointestinal diseases.2 H. pylori is present in the stomachs of roughly half the global population.3 Nevertheless, the precise mechanisms by which this microorganism is transmitted between individuals remain undefined.
H. pylori can be transmitted through various pathways, including iatrogenic means, fecal-oral contact, oral-oral contact, and via contaminated food and water.4 In recent decades, the rate at which H. pylori infection is eradicated has significantly declined. There is also a high likelihood of this rate dropping even further due to the increasing issue of drug resistance.5 The recurrence rate of H. pylori infection varies considerably, ranging from 0% to 41.5%.6 Evidence suggests that the highest incidence of infection recurrence occurs within the first year following treatment, strongly indicating that these cases are more likely attributable to re-infection rather than recrudescence.7H. pylori has been detected in various oral niches, including saliva, dental plaque, and gingival pockets. Research indicates a wide range in the reported prevalence of H. pylori in dental plaque, varying from 0% to 100% across different studies.8 Furthermore, genotypic analysis of H. pylori isolated from disparate sites reveals identical or highly similar strains, suggesting either the presence of a single species or genetic mutations within the same species.9,10
In research conducted by Hu et al11 and Gao et al,12 polymerase chain reaction (PCR) analysis consistently demonstrated that dental plaque served as the primary reservoir for this bacterium, with higher concentrations observed in subgingival plaque compared to supragingival plaque. Furthermore, the presence of urea within biofilms enhances the viability of urease-producing bacteria like H. pylori, with a synergistic relationship possibly accounting for the elevated prevalence of this bacterium in dental plaque relative to other oral sites.13
Multiple studies have indicated a positive correlation between the levels of dental plaque and oral hygiene, and the presence of H. pylori infection in both the oral cavity and the gastric system.14 The predilection of H. pylori for supragingival plaque can be attributed to its microaerophilic nature. H. pylori present in supragingival plaque can migrate to the subgingival plaque, with elevated levels of the bacterium observed in both locations in individuals with periodontal diseases.15 The persistence of H. pylori within the oral cavity is contingent upon its interactions with other microorganisms in the plaque biofilm. H. pylori demonstrates selective adhesion to certain bacterial species, including Fusobacterium nucleatum (F. nucleatum),16,17 Tannerella forsythia,18 and Porphyromonas gingivalis.19 Given the proliferation of these bacteria in individuals with periodontal disease, it is plausible that dental plaque in these patients serves as a reservoir for H. pylori, likely due to its interactions with these cohabiting bacterial species.8 Periodontal diseases may be linked to gastrointestinal infections and their recurrence; however, the precise involvement of the oral cavity in the transmission and relapse of these infections remains a subject of ongoing debate.
Triple therapy, the most common treatment for H. pylori infection,20-23 demonstrates limited efficacy in eradicating oral H. pylori.24-26 Since dental plaque is a bacterial biofilm, it effectively shields the resident microorganisms from systemic antimicrobial agents,8 thereby impeding antibiotic penetration into the biofilm structure.27 The diminished concentration of antibiotics in saliva, compared to gastric levels, consequently attenuates their efficacy even against microorganisms present in non-plaque environments, such as saliva itself.28
The presence of H. pylori in the oral cavity has been linked to a diminished efficacy of eradication therapy, potentially leading to treatment failure and recurrent gastrointestinal infections.8 Research has explored the impact of periodontal therapy on gastric H. pylori infections. For instance, Sheu et al29 proposed that individuals with periodontal disease may be more susceptible to H. pylori recurrence, even following successful eradication of the bacterium. Some researchers have suggested that integrating periodontal therapy with standard triple therapy significantly improves the eradication of gastrointestinal H. pylori infection. This combined approach is more effective than medication alone, culminating in a notably lower recurrence rate of the infection among patients who undergo periodontal therapy.30-32 The oral cavity’s potential to harbor and transmit gastrointestinal infections suggests that periodontal therapy could be a valuable intervention in treating gastric H. pylori infections.33-35
Given the ongoing debate surrounding the involvement of oral H. pylori in gastrointestinal infections and the effectiveness of periodontal therapy in eradicating gastric H. pylori, this study aimed to assess the efficacy of nonsurgical periodontal therapy in eradicating H. pylori infection.
Methods
This study, performed at the Department of Periodontics (Isfahan, Iran), received ethical approval from Isfahan University of Medical Sciences (Approval ID: IR.MUI.REC.1396.3.174) and was registered with the Iranian Registry of Clinical Trials (IRCT) (Approval ID: IRCT20150210021029N3). All participants provided written informed consent, and their involvement was voluntary.
Study design
The current single-center, double-masked, prospective, randomized clinical trial (RCT) was conducted with a parallel-group design. Figure 1 illustrates the methodological workflow. This study was a double-blind RCT, with a minimum of 25 patients in each of the study groups, with an 80% probability that a difference of 0.37 would be significant at the α = 0.05 level. Initially, 107 patients were recruited. Five participants subsequently dropped out, resulting in a final 102 patients. These patients, diagnosed with peptic ulcer or dyspepsia, tested positive for gastric infection via serology or biopsy. They were referred from the gastrointestinal clinic of Al-Zahra and provided informed consent before receiving treatment. The reporting of results conformed to the guidelines outlined in the 2010 Consolidated Standards of Reporting Trials (CONSORT) statement.
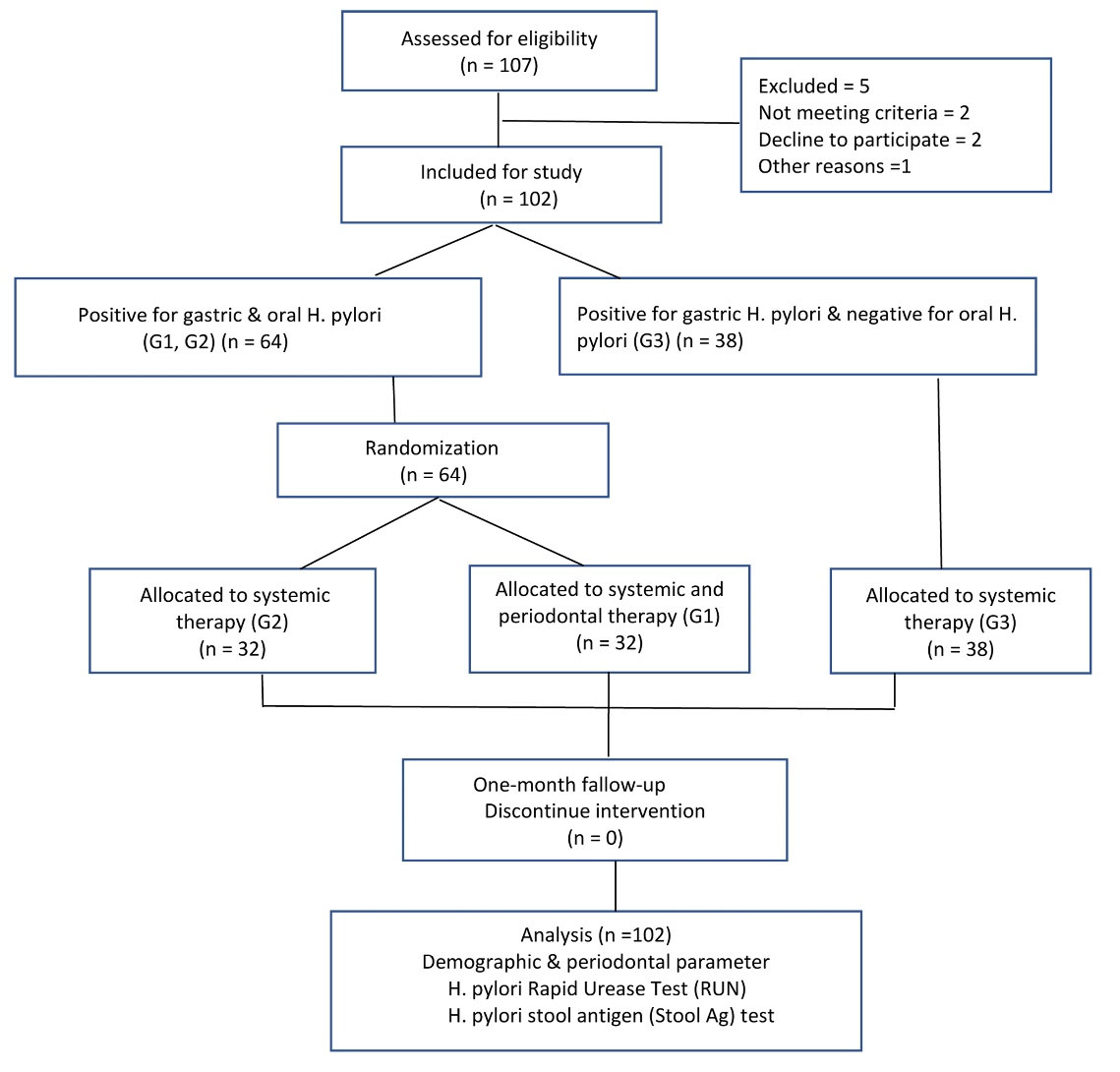
Figure 1.
Flow diagram of the study
.
Flow diagram of the study
Selection criteria
The inclusion criteria encompassed patients diagnosed with peptic ulcer or dyspepsia, who exhibited positive serological or biopsy results for gastric infection.
The exclusion criteria for this study included recent treatment for H. pylori within the preceding 2 months, the use of antibiotics or gastrointestinal medications (e.g., proton pump inhibitors) in the preceding 2 months, prior gastric surgery, and a history of periodontal therapy within the preceding 6 months. Upon commencing the study, each patient’s questionnaire was used to document demographic data, including age, gender, smoking status, and alcohol use. Subsequently, a comprehensive full-mouth periodontal examination was conducted for all participants. An investigator (Sh. R.) meticulously evaluated several periodontal indicators, including the plaque index, bleeding on probing, presence of calculus, coated tongue, and clinical probing depth.36 Subsequently, the subgingival plaque was analyzed for the presence of H. pylori using the rapid urease test (RUT).35 This diagnostic procedure, detailed elsewhere, was conducted using the RUT kit (Bahar Medical Laboratory, Tehran, Iran). Subgingival plaque was meticulously collected from 3‒4-mm periodontal pockets of the posterior teeth using a Williams periodontal probe. The collected plaque was then transferred into a tube containing 0.5 mL of solution. A positive test result was indicated by a color transformation of the solution from yellow to pink within 20 minutes.
While various oral microorganisms, including Streptococcus species, Haemophilus species, and Actinomyces species, possess the capacity for urea production, only H. pylori synthesizes urea in quantities substantial enough to induce a color change in solution within 20 minutes.20
Interventions
In a study involving 64 participants who tested positive for an RUT, individuals were randomly divided into two distinct groups using random allocation software. Group 1, designated as G1 (G + O + tP), comprised participants found to be positive for H. pylori in both gastric and oral samples. This group received a combination of triple eradication therapy and nonsurgical periodontal therapy. Group 2, designated as G2 (G + O + t), comprised participants who tested positive for H. pylori in both gastric and oral specimens and received triple eradication therapy exclusively. Additionally, 38 participants who yielded negative RUT results were allocated to group 3 [G3 (G + O - t)]; these individuals tested positive for H. pylori in gastric samples but were negative in oral samples and also underwent triple eradication therapy.
Treatment regimens
In all the three groups, the triple therapy regimen comprised 40 mg of pantoprazole administered twice daily, 240 mg of bismuth administered twice daily, 1 g of amoxicillin twice daily, and 500 mg of clarithromycin administered twice daily, sustained over 14 days. For G1, periodontal therapy involved comprehensive oral hygiene instruction and full-mouth disinfection (FMD). This FMD procedure encompassed a single-stage, complete scaling and root planing executed with an ultrasonic device, alongside the irrigation of periodontal pockets, the tongue, and the tonsils using a 0.2% chlorhexidine-gluconate solution (chlorhexidine-Najo, Tehran, Iran) concurrent with the triple therapy.
Following this, an 0.2% chlorhexidine gluconate mouthwash was prescribed twice daily for two weeks. Concurrently, periodontal therapy commenced alongside triple therapy. To assess the impact of these treatments on H. pylori eradication rates, a stool antigen (stool Ag) test was conducted at the Al-Zahra Hospital laboratory at least one month after treatment.
Outcomes
This study primarily aimed to evaluate the efficacy of a combined nonsurgical periodontal and systemic therapeutic approach compared to systemic therapy alone for eradicating H. pylori from the subgingival biofilm in patients harboring the bacterium.
Randomization and concealment
Sixty-four participants, all testing positive for RUT, were randomly allocated to one of two groups (G1 and G2) using random allocation software. G1 underwent systemic and nonsurgical periodontal therapy (G + O + tp), while G2 received systemic therapy only (G + O + t).
Blinding
The researcher responsible for outcome evaluation and statistical analysis was blinded.
Statistical analysis
Data analysis was conducted using SPSS 22. Statistical significance was defined at P < 0.05.
Results
In this study, 102 participants were enrolled, comprising 49 males (48%) and 53 females (52%). A chi-squared test revealed no significant difference in gender distribution across the groups (P = 0.236). Among the participants, 16.2% were identified as smokers. Fisher’s exact test revealed no significant difference in smoking prevalence across the three groups (P = 0.799). Additionally, 9.3% of participants reported alcohol use, with Fisher’s exact test indicating no significant differences across the three groups (P = 0.99). Dental calculus was observed in 44 patients (58.7%). A chi-squared test revealed no significant difference in the prevalence of dental calculus across the three study groups (P = 0.113). A coated tongue was present in 14 patients (18.7%). Fisher’s exact test indicated no significant difference in the occurrence of coated tongue across the three groups (P = 0.99). Furthermore, a Kruskal-Wallis test demonstrated no significant difference in the mean number of amalgam restorations across the three study groups (P = 0.839) (Table 1).
Table 1.
Demographic and clinical parameters of all study subjects
|
Demographic and clinical parameters
|
G1
*
|
G2
|
G3
|
P
value
|
| Male |
17 |
14 |
18 |
0.236 |
| Female |
18 |
19 |
16 |
| Smoker** |
8 |
6 |
7 |
0.799 |
| Alcohol user** |
2 |
2 |
3 |
0.99 |
| Calculus# |
18 |
15 |
11 |
0.113 |
| Coated tongue# |
4 |
5 |
5 |
0.99 |
| Amalgam## |
5.6 |
5.76 |
6.48 |
0.839 |
*Groups: G1 positive for H. pylori in both gastric and oral samples and received combined treatment; G2 positive for H. pylori in both gastric and oral specimens and received triple therapy; G3 positive for H. pylori in gastric but negative in oral samples and received triple therapy. **Number of smokers and alcoholic patients in each group. #Number of patients who have calculus or a coated tongue. ##Mean number of amalgam restorations in each group.
The mean plaque index measurements for the three groups were as follows: G1: 66.28 ± 15.83, G2: 63.61 ± 19.49, G3: 63.6 ± 18.6. The highest mean plaque index was observed in G1, while G2 exhibited the lowest. However, a one-way analysis of variance (ANOVA) revealed no significant difference in the mean plaque index across the three groups (P = 0.8).
The mean plaque index was similar across the groups with (64.72 ± 17.67) and without (63.6 ± 18.16) oral H. pylori. An independent samples t-test was employed to analyze these plaque index data between the two groups. The findings revealed no significant difference between the two groups, those with and those without oral H. pylori (P = 0.798). The mean probing depth measurements were 2.51 ± 0.8 in G1, 2.79 ± 1.13 in G2, and 2.62 ± 0.42 in G3.
The Kruskal-Wallis test results indicated no significant difference in the mean scores of the three groups (P = 0.669). Additionally, the mean probing depth was recorded as 2.654 mm in the group with oral H. pylori and 2.625 mm in the group without oral H. pylori. Consistent with these findings, the Mann-Whitney test revealed no significant difference in mean probing depth between individuals with and without oral H. pylori (P = 0.496).
In G1, one month after treatment, the RUT yielded negative results in 14 patients (56%), while the remaining 11 patients (44%) continued to test positive. A McNemar test was conducted to compare the primary and secondary RUT results, revealing a significant difference (P < 0.001).
One month following drug administration, positive stool Ag results were observed in 6 subjects from G1, 15 subjects from G2, and 6 subjects from G3. The corresponding eradication rates for these groups were 80%, 52%, and 84%, respectively (Figure 2).
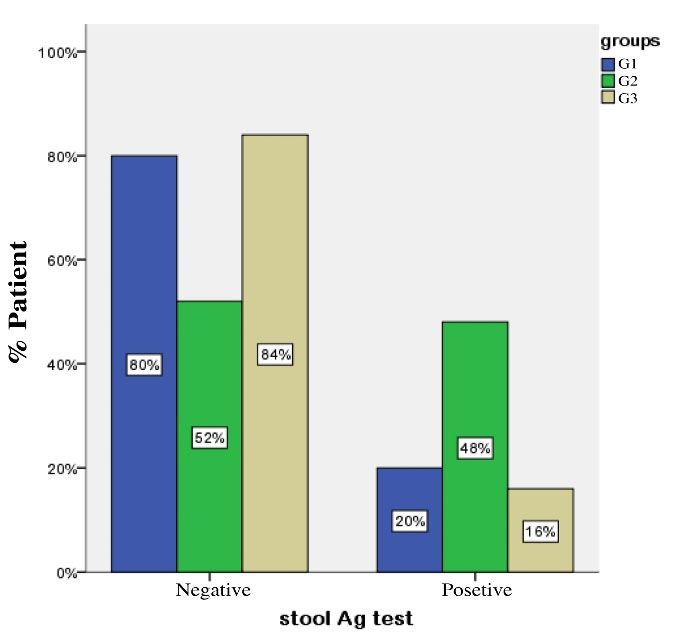
Figure 2.
Percentage of patients with negative or positive stool Ag test one month after treatment. G1: positive for H. pylori in both gastric and oral samples and received combined treatment, G2: positive for H. pylori in both gastric and oral samples and received triple therapy alone, G3: positive for H. pylori in gastric but negative in oral samples and received triple therapy. Stool Ag test: EIA based on enzyme immune assay
.
Percentage of patients with negative or positive stool Ag test one month after treatment. G1: positive for H. pylori in both gastric and oral samples and received combined treatment, G2: positive for H. pylori in both gastric and oral samples and received triple therapy alone, G3: positive for H. pylori in gastric but negative in oral samples and received triple therapy. Stool Ag test: EIA based on enzyme immune assay
A chi-squared test revealed significant differences between G1 and G2 (P =0.037) and between G2 and G3 (P =0.015). However, Fisher’s exact test results revealed no significant difference (P =1.000) between G1 and G3.
Discussion
This research aimed to evaluate the impact of nonsurgical periodontal therapy on the eradication of gastric H. pylori. Our findings indicated that 80% of patients receiving both systemic triple therapy and nonsurgical periodontal therapy achieved gastric eradication, whereas only 52% of patients undergoing systemic triple therapy alone demonstrated comparable results. The combined therapy group showed an enhanced clinical outcome, achieving statistical significance. This finding aligns with prior research,30,31,37,38indicating that periodontal therapy positively impacts the healing of gastrointestinal infections. However, this observation diverges from the conclusions drawn by Zaric et al34 and Song and Li.39 In Zaric et al’s study, the G + O + group exhibited elevated mean plaque index and probing depth compared to the G + O - group. However, this observed difference was not statistically significant (P = 0.07). These results imply that H. pylori may preferentially colonize newly formed plaque, while established or older plaque demonstrates less susceptibility to colonization.34 The current understanding suggests that the specific composition of dental plaque containing H. pylori holds greater significance than the sheer volume of plaque accumulation. Probing depth appears to be a potential risk factor for the presence of oral H. pylori, which is likely due to the interaction of H. pylori with enhanced periopathogens, such as F. nucleatum, in deeper periodontal pockets.16,17 The discrepancies observed between studies, despite similar sample sizes, may stem from variations in the methodologies employed for H. pylori detection. For instance, Zaricet al’s study used PCR and the urea breath test (UBT) to identify both oral and digestive H. pylori bacteria.34 In contrast, the current study relied on the RUT and stool Ag detection methods. H. pylori is frequently detected in dental plaque. Therefore, nonsurgical periodontal therapy, which involves the removal of plaque and calculus from tooth surfaces, may be a significant factor in both the eradication and prevention of gastrointestinal H. pylori re-infection.
As suggested by Sheu et al,29 periodontal disease may increase an individual’s susceptibility to recurrent H. pylori infection, even following successful eradication of the bacterium. Furthermore, studies by Gao et al12 and Butt et al31 have demonstrated that a combined approach of drug therapy and periodontal therapy is more effective in eradicating gastrointestinal H. pylori infections compared to drug therapy alone. Their research also suggests that individuals with oral microorganisms exhibit a higher rate of infection recurrence. Namiot et al33 presented findings that diverge from other research, indicating no improvement in the effectiveness of H. pylori eradication in the gastrointestinal tract through enhanced oral health and hygiene.This discrepancy may stem from two key factors: First, their intervention was limited to mechanical oral hygiene alongside drug therapy, and second, the study did not assess the presence of oral H. pylori in the participant groups.33
The differential success rates observed in H. pylori eradication—specifically, the efficacy of combined medication and periodontal therapy in patients with oral H. pylori, versus the success of drug therapy alone in those without oral H. pylori suggest a critical insight. These findings collectively imply that the presence of oral H. pylori may serve as a potential risk factor for the failure of conventional drug therapy aimed at eradicating H. pylori. Dental plaque serves as a natural host for H. pylori, potentially contributing to the recurrence of infections following gastric eradication therapy. Therefore, screening patients for H. pylori within dental plaque could aid in its elimination and reduce the development of antibiotic resistance. This finding aligns with studies indicating a concurrent eradication of H. pylori in both oral and gastric sites among patients.39,40
While systemic therapy effectively eradicates H. pylori from the stomach, the bacterium residing in dental plaque is shielded from systemic antibiotics by its protective biofilm. Consequently, if not mechanically removed, this dental plaque acts as a potential reservoir for gastric re-infection.
Certain research suggests a correlation between the existence and intensity of periodontal disease and the concentration of H. pylori in the oral cavity. Given the challenges in eradicating oral H. pylori through drug therapy and the potential for dental plaque to serve as a reservoir for infection recurrence, periodontal treatment may effectively diminish oral H. pylori levels. In contrast, drug therapy alone has not demonstrated a significant reduction. The presence of H. pylori within dental plaque suggests that disrupting this plaque through mechanical and chemical interventions is crucial for its eradication. Current research indicates that antimicrobial agents are unlikely to be effective against H. pylori in this context. The plaque’s structural integrity must first be disrupted for these agents to be effective.40 A limitation of this study is its limited sample size and brief follow-up period; thus, further research, ideally in the form of large-scale RCTs, is required to corroborate the efficacy of nonsurgical periodontal therapy in H. pylori management.
Conclusion
The current research demonstrated that while antibiotic therapy alone effectively eliminated gastrointestinal infections, incorporating periodontal therapy into standard therapies for oral H. pylori patients more significantly reduced both oral and gastrointestinal H. pylori load. Given that the periodontal pocket serves as a primary reservoir for H. pylori, the observed effect likely stems from a reduction in both plaque accumulation and probing pocket depth.
Competing Interests
The authors declare that they have no competing interests.
Consent for Publication
Not applicable.
Data Availability Statement
All data regarding the methodology of the manuscript have been shared.
Ethical Approval
The present study was approved by the Ethics Committee of Isfahan University of Medical Sciences (Code: ID: IR.MUI.REC.1396.3.174).
Acknowledgements
We gratefully acknowledge the continuous guidance and support provided by the Vice-Chancellor for Research at Isfahan University of Medical Sciences.
References
- Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006; 19(3):449-90. doi: 10.1128/cmr.00054-05 [Crossref] [ Google Scholar]
- FitzGerald R, Smith SM. An overview of Helicobacter pylori infection. Methods Mol Biol 2021; 2283:1-14. doi: 10.1007/978-1-0716-1302-3_1 [Crossref] [ Google Scholar]
- Eyigor M, Eyigor H, Gultekin B, Aydin N. Detection of Helicobacter pylori in adenotonsiller tissue specimens by rapid urease test and polymerase chain reaction. Eur Arch Otorhinolaryngol 2009; 266(10):1611-3. doi: 10.1007/s00405-008-0903-6 [Crossref] [ Google Scholar]
- Duan M, Li Y, Liu J, Zhang W, Dong Y, Han Z. Transmission routes and patterns of Helicobacter pylori. Helicobacter 2023; 28(1):e12945. doi: 10.1111/hel.12945 [Crossref] [ Google Scholar]
- Ng HY, Leung WK, Cheung KS. Antibiotic resistance, susceptibility testing and stewardship in Helicobacter pylori infection. Int J Mol Sci 2023; 24(14):11708. doi: 10.3390/ijms241411708 [Crossref] [ Google Scholar]
- Niv Y. H. pylori recurrence after successful eradication. World J Gastroenterol 2008; 14(10):1477-8. doi: 10.3748/wjg.14.1477 [Crossref] [ Google Scholar]
- Gisbert JP, Luna M, Gómez B, Herrerías JM, Monés J, Castro-Fernández M. Recurrence of Helicobacter pylori infection after several eradication therapies: long-term follow-up of 1000 patients. Aliment Pharmacol Ther 2006; 23(6):713-9. doi: 10.1111/j.1365-2036.2006.02827.x [Crossref] [ Google Scholar]
- Anand PS, Kamath KP, Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J Gastroenterol 2014; 20(19):5639-53. doi: 10.3748/wjg.v20.i19.5639 [Crossref] [ Google Scholar]
- Silva DG, Stevens RH, Macedo JM, Albano RM, Falabella ME, Veerman EC. Detection of cytotoxin genotypes of Helicobacter pylori in stomach, saliva and dental plaque. Arch Oral Biol 2009; 54(7):684-8. doi: 10.1016/j.archoralbio.2009.04.006 [Crossref] [ Google Scholar]
- Fernández-Tilapa G, Axinecuilteco-Hilera J, Giono-Cerezo S, Martínez-Carrillo DN, Illades-Aguiar B, Román-Román A. vacA genotypes in oral cavity and Helicobacter pylori seropositivity among adults without dyspepsia. Med Oral Patol Oral Cir Bucal 2011; 16(2):e175-80. doi: 10.4317/medoral.16.e175 [Crossref] [ Google Scholar]
- Hu W, Cao C, Meng H, Zhang J, Ma D, Zhang L. Detection and analysis of Helicobacter pylori in oral cavity and stomach from chronic gastritis patients. Zhonghua Yi Xue Za Zhi 2002; 82(15):1037-41. [ Google Scholar]
- Gao J, Li Y, Wang Q, Qi C, Zhu S. Correlation between distribution of Helicobacter pylori in oral cavity and chronic stomach conditions. J Huazhong Univ Sci Technolog Med Sci 2011; 31(3):409-12. doi: 10.1007/s11596-011-0391-6 [Crossref] [ Google Scholar]
- Sousa C, Ferreira R, Santos SB, Azevedo NF, Melo LDR. Advances on diagnosis of Helicobacter pylori infections. Crit Rev Microbiol 2023; 49(6):671-92. doi: 10.1080/1040841x.2022.2125287 [Crossref] [ Google Scholar]
- Zhang L, Chen X, Ren B, Zhou X, Cheng L. Helicobacter pylori in the oral cavity: current evidence and potential survival strategies. Int J Mol Sci 2022; 23(21):13646. doi: 10.3390/ijms232113646 [Crossref] [ Google Scholar]
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000 2002; 28:12-55. doi: 10.1034/j.1600-0757.2002.280102.x [Crossref] [ Google Scholar]
- Ishihara K, Miura T, Kimizuka R, Ebihara Y, Mizuno Y, Okuda K. Oral bacteria inhibit Helicobacter pylori growth. FEMS Microbiol Lett 1997; 152(2):355-61. doi: 10.1111/j.1574-6968.1997.tb10452.x [Crossref] [ Google Scholar]
- Andersen RN, Ganeshkumar N, Kolenbrander PE. Helicobacter pylori adheres selectively to Fusobacterium spp. Oral Microbiol Immunol 1998; 13(1):51-4. doi: 10.1111/j.1399-302x.1998.tb00751.x [Crossref] [ Google Scholar]
- Umeda M, Kobayashi H, Takeuchi Y, Hayashi J, Morotome-Hayashi Y, Yano K. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol 2003; 74(1):129-34. doi: 10.1902/jop.2003.74.1.129 [Crossref] [ Google Scholar]
- Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001; 6(3):254-61. doi: 10.1046/j.1523-5378.2001.00037.x [Crossref] [ Google Scholar]
- Hunt RH, Fallone CA, Thomson AB. Canadian Helicobacter pylori consensus conference update: infections in adults. Can J Gastroenterol 1999; 13(3):213-7. doi: 10.1155/1999/180751 [Crossref] [ Google Scholar]
- Shirin H, Birkenfeld S, Shevah O, Levine A, Epstein J, Boaz M. Application of Maastricht 2-2000 guidelines for the management of Helicobacter pylori among specialists and primary care physicians in Israel: are we missing the malignant potential of Helicobacter pylori?. J Clin Gastroenterol 2004; 38(4):322-5. doi: 10.1097/00004836-200404000-00005 [Crossref] [ Google Scholar]
- Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol 2007; 21(2):315-24. doi: 10.1016/j.bpg.2006.11.001 [Crossref] [ Google Scholar]
- Chey WD, Howden CW, Moss SF, Morgan DR, Greer KB, Grover S. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2024; 119(9):1730-53. doi: 10.14309/ajg.0000000000002968 [Crossref] [ Google Scholar]
- Czesnikiewicz-Guzik M, Loster B, Bielanski W, Guzik TJ, Konturek PC, Zapala J. Implications of oral Helicobacter pylori for the outcome of its gastric eradication therapy. J Clin Gastroenterol 2007; 41(2):145-51. doi: 10.1097/01.mcg.0000225654.85060.3d [Crossref] [ Google Scholar]
- Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter 2000; 5(1):30-7. doi: 10.1046/j.1523-5378.2000.00004.x [Crossref] [ Google Scholar]
- Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta-analysis. J Oral Pathol Med 2011; 40(4):317-24. doi: 10.1111/j.1600-0714.2011.01006.x [Crossref] [ Google Scholar]
- Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 1996; 111(2):358-67. doi: 10.1053/gast.1996.v111.pm8690200 [Crossref] [ Google Scholar]
- Wüst J, Hardegger U. Penetration of clarithromycin into human saliva. Chemotherapy 1993; 39(5):293-6. doi: 10.1159/000239139 [Crossref] [ Google Scholar]
- Sheu BS, Cheng HC, Yang YJ, Yang HB, Wu JJ. The presence of dental disease can be a risk factor for recurrent Helicobacter pylori infection after eradication therapy: a 3-year follow-up. Endoscopy 2007; 39(11):942-7. doi: 10.1055/s-2007-966787 [Crossref] [ Google Scholar]
- Ren Q, Yan X, Zhou Y, Li WX. Periodontal therapy as adjunctive treatment for gastric Helicobacter pylori infection. Cochrane Database Syst Rev 2016; 2(2):CD009477. doi: 10.1002/14651858.CD009477.pub2 [Crossref] [ Google Scholar]
- Butt AK, Khan AA, Khan AA, Izhar M, Alam A, Shah SW. Correlation of Helicobacter pylori in dental plaque and gastric mucosa of dyspeptic patients. J Pak Med Assoc 2002; 52(5):196-200. [ Google Scholar]
- Jia CL, Jiang GS, Li CH, Li CR. Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. J Periodontol 2009; 80(10):1606-9. doi: 10.1902/jop.2009.090029 [Crossref] [ Google Scholar]
- Namiot DB, Namiot Z, Kemona A, Bucki R, Gotebiewska M. Oral health status and oral hygiene practices of patients with peptic ulcer and how these affect Helicobacter pylori eradication from the stomach. Helicobacter 2007; 12(1):63-7. doi: 10.1111/j.1523-5378.2007.00471.x [Crossref] [ Google Scholar]
- Zaric S, Bojic B, Jankovic L, Dapcevic B, Popovic B, Cakic S. Periodontal therapy improves gastric Helicobacter pylori eradication. J Dent Res 2009; 88(10):946-50. doi: 10.1177/0022034509344559 [Crossref] [ Google Scholar]
- Tsimpiris A, Grigoriadis A, Tsolianos I, Moschos I, Goulis DG, Kouklakis G. Periodontitis and Helicobacter pylori infection: eradication and periodontal therapy combination. Eur J Dent 2022; 16(1):145-52. doi: 10.1055/s-0041-1731928 [Crossref] [ Google Scholar]
- Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38(6):610-6. doi: 10.1902/jop.1967.38.6.610 [Crossref] [ Google Scholar]
- Yuksel Sert S, Ozturk A, Bektas A, Cengiz MI. Periodontal treatment is more effective in gastric Helicobacter pylori eradication in those patients who maintain good oral hygiene. Int Dent J 2019; 69(5):392-9. doi: 10.1111/idj.12484 [Crossref] [ Google Scholar]
- Tongtawee T, Wattanawongdon W, Simawaranon T. Effects of periodontal therapy on eradication and recurrence of Helicobacter pylori infection after successful treatment. J Int Med Res 2019; 47(2):875-83. doi: 10.1177/0300060518816158 [Crossref] [ Google Scholar]
- Song HY, Li Y. Can eradication rate of gastric Helicobacter pylori be improved by killing oral Helicobacter pylori?. World J Gastroenterol 2013; 19(39):6645-50. doi: 10.3748/wjg.v19.i39.6645 [Crossref] [ Google Scholar]
- Herrera D, Alonso B, León R, Roldán S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol 2008; 35(8 Suppl):45-66. doi: 10.1111/j.1600-051X.2008.01260.x [Crossref] [ Google Scholar]