J Adv Periodontol Implant Dent. 16(2):116-122.
doi: 10.34172/japid.2024.017
Research Article
Effect of atorvastatin gel in non-surgical treatment of peri-implant mucositis: A randomized controlled clinical trial
Azin Khorramdel Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing, 1 
Katayoun Mogharrab Alile Conceptualization, Data curation, Formal analysis, Investigation, Resources, Software, Writing – original draft, Writing – review & editing, 2, * 
Yousef Kananizadeh Conceptualization, Supervision, Writing – review & editing, 3 
Seyed Amin Mousavi Investigation, Project administration, Supervision, Visualization, Writing – review & editing, 4 
Fatima Molavi Resources, Writing – review & editing, 5 
Author information:
1Department of Periodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
2Dentist, Faculty of Dentistry, Tabriz Medical Sciences, Islamic Azad University, Tabriz, Iran
3Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Tabriz Medical Sciences, Islamic Azad University, Tabriz, Iran
4Department of Prosthodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background.
Peri-implant diseases, such as peri-implant mucositis and peri-implantitis, are inflammatory conditions caused by biofilms that can lead to the loss of surrounding soft tissues and bone. The most effective treatment involves non-surgical mechanical debridement to remove plaque, but other treatment modalities have shown limited success. This study investigated the anti-inflammatory and immunomodulatory effects of atorvastatin (ATV) gel as an additional treatment for peri-implant mucositis.
Methods.
In this double-masked, randomized clinical trial, 49 patients with peri-implant mucositis were randomly divided into two treatment groups: mechanical debridement (MD)+placebo or MD+ATV gel. At baseline, 1 month, and 3 months after the intervention, periodontal parameters, including probing depth (PD), bleeding on probing (BOP), clinical attachment level (CAL), and pain on probing (POP), were measured. Data were analyzed using independent t-test and paired t-test.
Results.
Statistically significant improvements in CAL and POP were observed from baseline to each time point throughout the study period (P≤0.001). PD and BOP were statistically significant 1 month and 3 months after the intervention, respectively (P<0.05).
Conclusion.
The clinical parameters associated with peri-implant mucosal inflammation further improved when ATV gel was used with MD.
Keywords: Atorvastatin, Clinical trial, Dental implants, Mucositis
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Dental implant therapy is a popular method for replacing missing teeth; however, it can lead to technical and biological complications known as peri-implant diseases.1 These biofilm-induced inflammations affect soft and hard bone tissues around osseointegrated implants. There are two categories of peri-implant diseases: peri-implant mucositis and peri-implantitis.2 Peri-implant mucositis is a reversible inflammation of the mucosa around the implant,3 while peri-implantitis involves progressive bone loss.4 The prevalence of peri-implant mucositis ranges from 23.9% to 88%, and peri-implantitis varies from 8.9% to 45%.5,6 Although plaque accumulation is the main cause,7 other risk factors include smoking, history of periodontitis, lack of regular periodontal maintenance, diabetes, implant design or surface characteristics, and excess cement.8,9
The standard approach for treating peri-implant mucositis is non-surgical treatment, which involves reinforcing oral hygiene practices, including professional and patient-administered plaque control techniques to mechanically remove microbial plaques from the implant surfaces. Studies have investigated adjunctive or alternative methods and non-surgical mechanical debridement for treating peri-implant mucositis.10 These methods include antimicrobial photodynamic therapy, antiseptics, topical or systemic antibiotics, abrasive devices, laser therapy, home care mouthwashes, and probiotics.11-14 However, it should be emphasized that regardless of the treatment used, adequate plaque control is important for the complete resolution of the condition.15
Statins, commonly prescribed for lower lipid levels to prevent cardiovascular events, have shown potential for treating periodontal diseases. Studies have demonstrated that statins can reduce tooth mobility, tooth loss, and bone resorption in patients with chronic periodontitis. In addition to their lipid-lowering effects, statins possess anti-inflammatory, immunomodulatory, antioxidant, antithrombotic, and endothelium-stabilizing properties. They can also promote angiogenesis and stimulate bone formation.16,17 Recent studies have shown that patients receiving statin treatment for chronic periodontitis have fewer pathological periodontal pockets than those not receiving such medication. Atorvastatin (ATV), in particular, demonstrates inhibitory effects on inflammatory cells and matrix metalloproteinases that play a crucial role in degrading connective tissue in periodontal diseases.16
Akram et al.18 found that 1.2% ATV gel applied locally improved clinical and radiographic parameters significantly. ATV was more effective than other statins, such as simvastatin, pravastatin, lovastatin, and fluvastatin, in lowering low-density lipoprotein. According to this study, ATV may be more effective than simvastatin in promoting bone regeneration in periodontal defects, reducing probing depth (PD) and clinical attachment level (CAL), and exerting anti-inflammatory effects.17,19 Although none of the recent studies demonstrated the effectiveness of ATV gel once it is used locally to treat peri-implant mucositis, the present study was undertaken to evaluate the effectivity of 1.2% ATV gel in addition to mechanical debridement for the treatment of peri-implant mucositis. This study was necessary as statins are very effective in anti-inflammatory effects, and a limited number of studies have been conducted in this field.
Methods
Study design
In this double-blinded randomized controlled clinical trial, 49 patients (20 males and 29 females, aged 40–60 years) diagnosed with peri-implant mucositis were selected from patients referred to a private periodontal office and the Faculty of Dentistry, Tabriz University of Medical Sciences. The patients were blinded to the type of treatment they received randomly (ATV or placebo gel), and the examiner was unaware of the patients’ allocation to the test or control groups. The researcher was aware of the interventions administered.
The study protocol was initially approved by the Institutional Ethics Committee of the Islamic Azad University of Medical Sciences Tabriz Branch (protocol number IR.IAU.TABRIZ.REC.1401.198) and was conducted in compliance with the Helsinki Declaration of 1975, as revised in 2013. The study findings were reported according to the 2010 CONSORT guidelines.
The study was officially registered with the local World Health Organization Registry Network under the code IRCT20220510054805N1. Following ethical approval, all the individuals were duly informed verbally, and written informed consent was obtained for their participation in the study.
Inclusion criteria
-
Age ≥ 18 years
-
Severe peri-implant mucositis or mild peri-implantitis (presence of bleeding on probing [BOP], PD > 3 mm, no soft tissue recession with or without minimal crestal bone loss ≤ 2 mm on periapical radiographs)
-
Functioning implants for ≥ 1 year20
Exclusion Criteria
-
Pregnancy and breastfeeding
-
Current and former smokers
-
Uncontrolled diabetes mellitus and immune-compromised systemic diseases
-
Presence of active periodontal disease after primary treatment
-
Use of systemic or topical antibiotics during the previous 3 months
-
Regular use of anti-inflammatory drugs in the last 3 months
-
Use of bisphosphonate, phenytoin, calcium antagonists, cyclosporine, comedo, warfarin, and heparin
-
Radiation to the head and neck region
-
History of allergy to the statin group of drugs
-
Statin therapy
-
Abuse of alcohol and drugs
-
Failing or refusing to sign an informed consent form20
Patient grouping
Forty-nine patients were selected based on the selection criteria, and after enrollment by an examiner, the patients were randomly allocated to either the test or control group. The randomization method was simple randomization and conducted using the RandomIZE Randomization tool app. The sample size was estimated to compare the average of the two groups from the respective formula with 95% confidence and 80% power, and an effect size equivalent to that of a similar study by Pradeep et al21 equaled 21 participants in each group. Owing to the existence of three stages of follow-up and the possibility of dropping samples by 30%, the final sample size increased to 27 people in each group and 54 in total. Throughout all analyses of the research findings, the investigators were not part of or aware of the randomization process.
After mechanical debridement in both groups, 1.2% ATV gel (1.2 mg/0.1 mL) was injected into the pockets around the implant in the test (ATV) group and placebo gel in the control (placebo) group. Mechanical debridement was performed using a plastic curette at the baseline for each patient. Prepared 1.2% ATV gel (1.2 mg/0.1 mL) or placebo gel was injected into the pockets around the implant using an insulin syringe in the test and placebo groups (Figure 1). Cyanoacrylate tissue adhesive was used to protect the area. After treatment completion, the patients were not prescribed antibiotics or anti-inflammatory drugs. They were given specific instructions for a week, including refraining from chewing hard or sticky food, brushing near the treated areas, and using any interdental aid. At one and three months after the intervention, all clinical parameters were measured again in both groups in the same area.
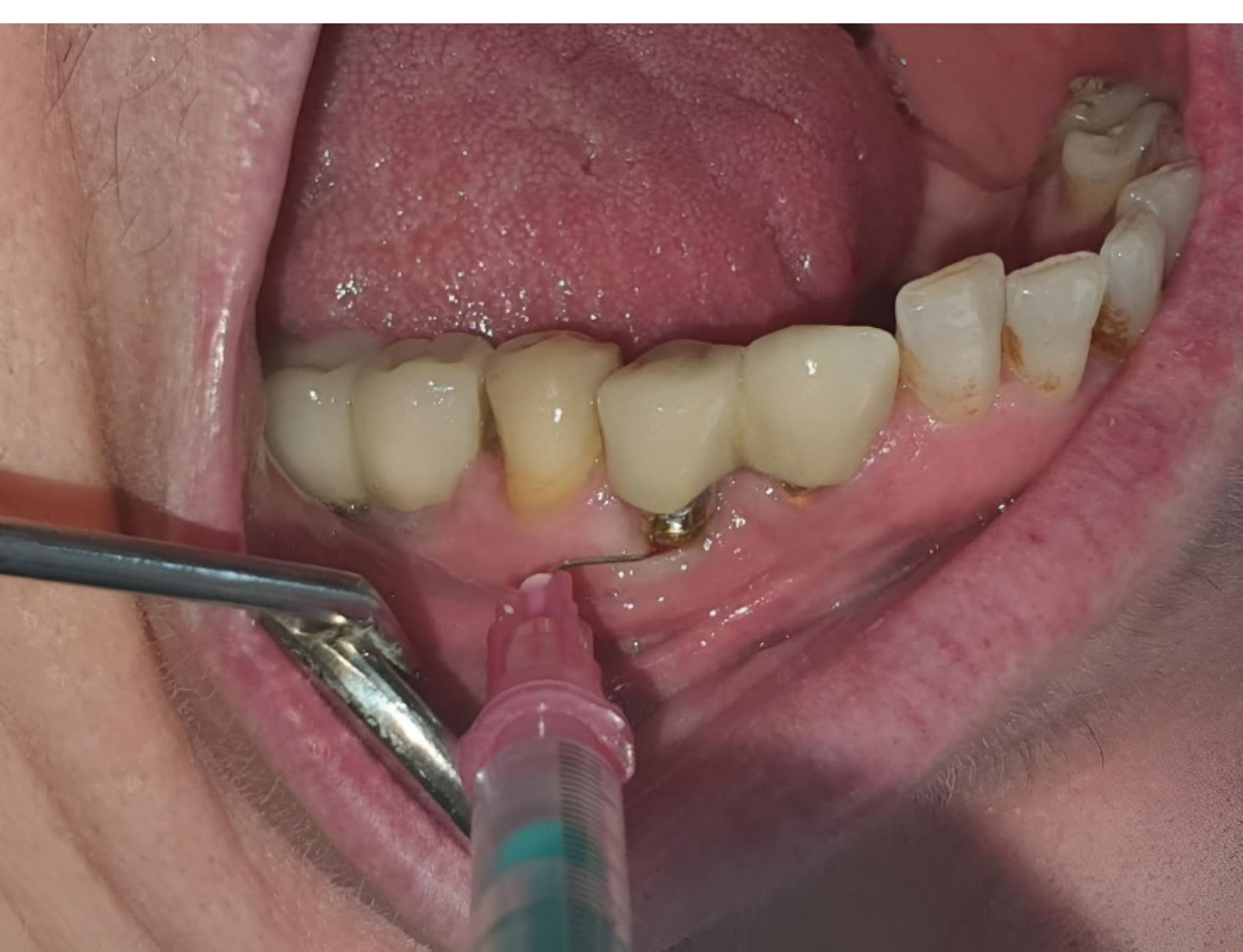
Figure 1.
Injections of 1.2% atorvastatin or placebo gel into the pockets surrounding the implant using an insulin syringe
.
Injections of 1.2% atorvastatin or placebo gel into the pockets surrounding the implant using an insulin syringe
Clinical evaluation
The evaluation involved recording various clinical parameters such as BOP, PD, CAL, and pain on probing (POP) at different time points: baseline (before mechanical debridement), 1 month, and 3 months. A custom-made acrylic stent and a color-coded periodontal probe (UNC-15, Hu-Friedy, Chicago, IL, USA) were used to ensure uniformity in measuring these parameters. An examiner blinded to each individual’s treatment recorded all the pre- and post-treatment clinical parameters.
Formulation of 1.2% ATV gel
ATV gel was prepared by a pharmacist using standard methods described in pharmacology texts.22-24 After intensive in vitro investigations for optimization and stability to prepare a multiple-dose solution of isotone and sterile ATV, the gel base was first prepared with sodium carboxymethyl cellulose (2.6%) and mannitol (9%), and the pharmaceutical stock solution was prepared at a concentration of 2% in propylene glycol solvent. Subsequently, at a 4:1 ratio, the drug solution was slowly added to the gel base. If opaque, 0.5% polysorbate 80 was added to the solution to increase the solubility of the drug. Finally, 1% benzyl alcohol was added to the solution as a preservative for injectable products. All steps were performed under laminar hood and aseptic conditions. The obtained solution was stored in sterile 1.5 mL of polyethylene microtubes and was stable at 2-8 °C for up to 1 month (Figure 2).
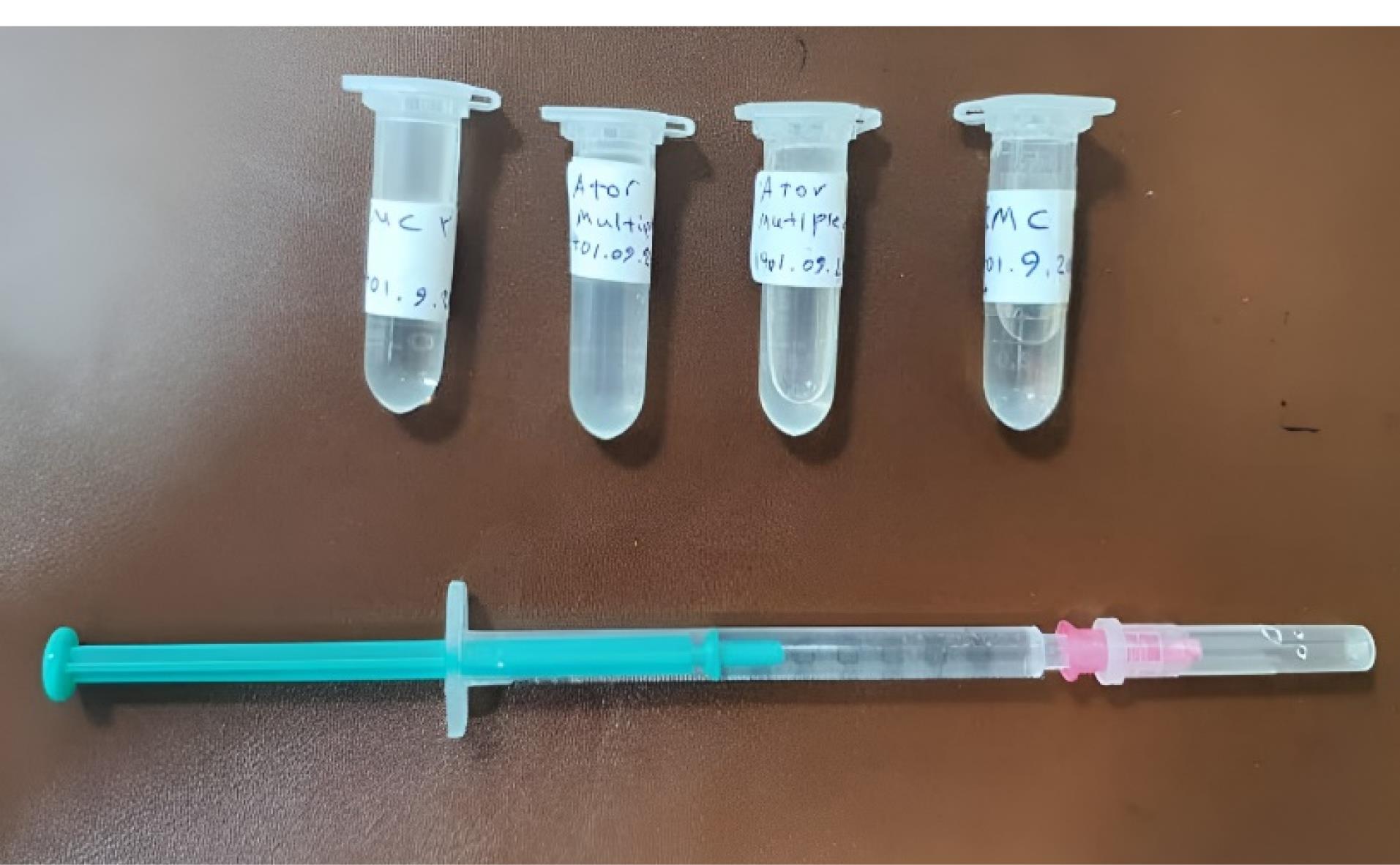
Figure 2.
1.2% ATV gel and placebo. Due to the non-availability of atorvastatin gel in the pharmaceutical market in Iran, a pharmacist formulated the atorvastatin gel
.
1.2% ATV gel and placebo. Due to the non-availability of atorvastatin gel in the pharmaceutical market in Iran, a pharmacist formulated the atorvastatin gel
Primary and secondary outcomes
The primary outcome of this study was the BOP. The secondary outcomes included PD, CAL, and POP.
Statistical analysis
SPSS 22 was used for the data analysis. Descriptive statistics (frequency, frequency percentage, mean, and standard deviation) and inferential statistics (chi-squared test, Fisher’s exact test, and independent t test) were used for data analysis. An independent t test was used to compare the results between the two groups, and a paired t test was used to compare intragroup results. Statistical significance was set at P < 0.05.
Results
Descriptive results
Forty-nine participants (one site/patient) out of 54 successfully concluded the study. Unfortunately, 5 individuals (2 in the ATV group and 3 in the placebo group) could not participate in the follow-up sessions (Figure 3). Thus, only 49 patients (20 men and 29 women) aged 41–60 years were included in the data analyses after completing the 3-month follow-up.
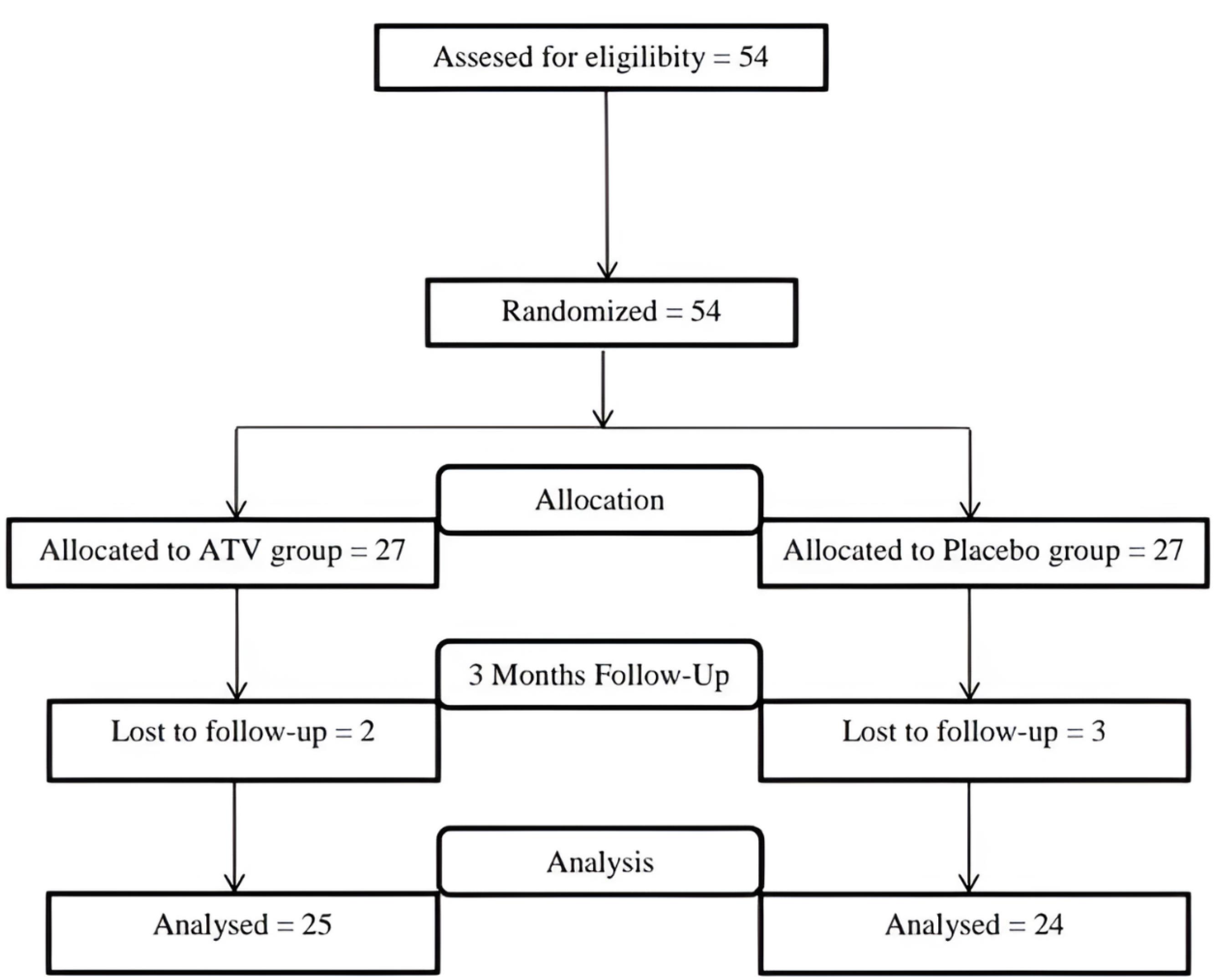
Figure 3.
CONSORT diagram
.
CONSORT diagram
Clinical parameters
Inter-group results
The clinical parameters (BOP, PD, CAL, and POP frequency distributions) at both the baseline and follow-up visits are shown in Tables 1 and 2. After one month, the independent t test showed no significant difference (P > 0.05) in the BOP index between the control and test groups. However, it significantly decreased (P < 0.001) in the test group after three months. There was no significant difference (P > 0.05) in PD between the two groups after three months, but it was significant one month after the intervention (P < 0.001). The CAL and POP variables showed significant differences 1 and 3 months after the intervention (P < 0.001).
Table 1.
Intergroup comparisons of parameters (Mean ± SD) at baseline and 1- and 3-month follow-up visits
|
Clinical parameters
|
Control group
|
Test group
|
|
Baseline
|
1 month
|
3 months
|
Baseline
|
1 month
|
3 months
|
| BOP (%) |
76.6 ± 15.2 |
24.4 ± 10.2 |
29.4 ± 9.4 |
79.4 ± 13.2 |
28.2 ± 8.9 |
49.1 ± 15.4 |
| PD (mm) |
5.4 ± 0.7 |
3.9 ± 0.9 |
3.6 ± 0.8 |
5.4 ± 0.6 |
3.2 ± 0.8 |
3.4 ± 0.6 |
| CAL (mm) |
6.0 ± 0.7 |
5.5 ± 0.9 |
5.9 ± 0.6 |
6.9 ± 0.8 |
4.1 ± 0.6 |
4.6 ± 0.5 |
| POP (cm) |
5.5 ± 1.7 |
4.7 ± 0.7 |
5.6 ± 1.7 |
4.5 ± 1.4 |
3.8 ± 1.1 |
3.6 ± 1.0 |
BOP: bleeding on probing; PD: pocket depth; CAL: clinical attachment level; POP: pain on probing.
Table 2.
Intergroup comparisons of parameters (P value) at baseline and follow-up visits
|
Parameters
|
Baseline (
P
)
|
One-month follow-up (
P
)
|
Three-month follow-up (
P
)
|
| BOP |
0.499 |
0.171 |
0.0001 |
| PD |
0.74 |
0.004 |
0.252 |
| CAL |
0.0001 |
0.0001 |
0.0001 |
| POP |
0.478 |
0.001 |
0.001 |
BOP: bleeding on probing; PD: pocket depth; CAL: clinical attachment level; POP: pain on probing.
Intra-group results
A comparison of intra-group results using a paired t-test showed a significant difference in PD one and three months after the intervention (P < 0.001). No significant difference (P > 0.05) was found in CAL in the control group at baseline and one and three months after the intervention. However, within the test group, the difference was statistically significant (P < 0.001). A comparison of the BOP results showed a significant difference (P < 0.001) one and three months after the intervention. The POP results were significantly different (P ≤ 0.05) one month after the intervention, both within the control and test groups. There was no significant difference (P > 0.05) in the control group three months after the intervention, but the difference was significant (P < 0.05) in the test group (Table 3).
Table 3.
Intra-group comparisons of parameters at baseline and 1- and 3-month follow-up visits
|
Clinical parameters
|
Control group
|
Test group
|
|
One month from baseline
|
Three months from baseline
|
One month from baseline
|
Three months from baseline
|
|
(
P
)
|
(
P
)
|
| BOP (%) |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
| PD (mm) |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
| CAL (mm) |
0.067 |
0.426 |
0.0001 |
0.0001 |
| POP (cm) |
0.026 |
0.862 |
0.050 |
0.004 |
BOP: bleeding on probing; PD: pocket depth; CAL: clinical attachment level; POP: pain on probing.
Discussion
Statins have antimicrobial activity against periodontal pathogens and exhibit anti-inflammatory and immunomodulatory effects.25 Animal and clinical studies have supported the idea that statins can be used as an adjunctive treatment to scaling and root planing (SRP) to manage periodontal disease, including chronic periodontitis.18,26 Numerous studies have investigated the effectiveness of statins on various clinical parameters of periodontitis.27 Due to the pleiotropic (cholesterol-independent) effects of statins, such as immunomodulatory and anti-inflammatory effects, they are expected to improve periodontal clinical outcomes.28 Several studies have reported positive clinical effects, such as reduced PD, CAL, and BOP, with local administration of statins. Therefore, statins are considered a valuable adjunct to non-surgical and surgical treatments for periodontal disease.18,26,27
The current study evaluated the clinical efficacy of 1.2% ATV gel as a supplement to mechanical debridement in treating peri-implant mucositis. Compared with the placebo gel, the results revealed a significant improvement in clinical parameters. To the best of our knowledge, no study has directly compared the use of 1.2% ATV gel in treating peri-implant mucositis.
In contrast, Saxlin et al29 investigated the dual effects of statins on the periodontium. Their study revealed that statin use was associated with a higher risk of deep periodontal pockets in individuals without BOP. However, Kumari et al30 discovered that using a local 1.2% ATV gel significantly improved clinical and radiographic parameters compared with a placebo gel. Similar results were also reported by Lindy et al,31 who found that ATV or simvastatin led to 37% fewer pathological periodontal pockets than in the control group. In addition, animal models have suggested that statins have a beneficial impact on ligature-induced alveolar bone loss.32 In the current study, BOP significantly decreased from baseline to three months, indicating that ATV may have anti-inflammatory properties.
In the current study, no significant difference (P > 0.05) was observed in the BOP index between the control and test groups at the one-month follow-up. However, after three months, the difference was significant (P < 0.001) in the test group. However, the results for the PD variable one month after the intervention were significant (P < 0.001). There was a significant difference in CAL and POP between the control and test groups one and three months after the intervention (P < 0.001).
Studies using local statins have reported significant improvements in clinical periodontal outcomes compared with those using SRP. The subgingival release of statins allows for high concentrations and low doses of drugs in the periodontal tissues, leading to high patient acceptance and the ability to control the long-term release of therapeutic agents at the target sites without causing systemic side effects.33 Compared with oral administration, which results in rapid absorption and entry of the drug into the circulation, local application of the drug is preferred.34,35 Therefore, it is safer to administer the drug locally, and clinical results have demonstrated that it improves chronic periodontitis.27
Bertl et al26 found that the type of statin used was associated with periodontal outcomes. One study showed that rosuvastatin was the most effective, whereas another reported statistically significant effects of ATV. In two clinical trials evaluating the application of statins as an adjunct to SRP, rosuvastatin produced the best results regarding clinical and radiographic parameters such as PD reduction, CAL gain, and radiographic defect fill.27 The superior clinical advantages of rosuvastatin over ATV may be attributed to its stronger anti-inflammatory effect, which results in a greater reduction in C-reactive protein levels.36,37
Simvastatin is the most commonly used statin in clinical trials and is administered locally at a concentration of 1.2%. Numerous studies have shown significant improvements in clinical and radiographic results when using simvastatin.27 Retrospective studies have also shown that patients with severe chronic periodontitis who were treated with simvastatin or ATV had lower PD indices than those who did not receive statins.38 In addition, a recent study by Fajardo et al39 indicated that ATV may reduce alveolar bone loss and tooth mobility in individuals with periodontal disease. Goes et al40 reported that ATV could prevent alveolar bone loss in rats with ligature-induced periodontitis.
Pradeep et al21 evaluated the use of 1.2% ATV gel as a supplement to SRP for treating suprabony defects in patients with chronic periodontitis. The ATV group showed a significant reduction in clinical parameters such as BOP, PD, and CAL at the 3-, 6-, and 9-month follow-ups compared to the placebo group, indicating the anti-inflammatory effect of ATV.
It is recommended that more samples be used in clinical studies and that longer follow-up periods and investigations of inflammatory biomarkers be conducted.
Conclusion
Overall, the clinical parameters of the peri-implant mucosa improved using 1.2% ATV gel as an adjunct to mechanical debridement. The results of this study support the additional application of ATV gel for the treatment of peri-implant mucositis. By injecting 1.2% ATV gel into the pockets around implants with peri-implant mucositis, this clinical trial showed that it significantly reduced BOP, PD, POP, and CAL gain when used with mechanical debridement, compared with placebo gel. This may provide a new approach for treating the inflammation caused by peri-implant mucositis. The results of this study must be confirmed in long-term, multicenter, randomized, controlled clinical trials.
Acknowledgments
The authors used Cloud.trinka.ai to check grammar and refine the presentation of crucial information in the introduction and discussion sections.
Competing Interests
The authors declare that they have no competing interests.
Consent for Publication
Not applicable.
Data Availability Statement
The data from the reported study are available upon request from the corresponding author.
Ethical Approval
The study was approved by the Institutional Ethical Committee of the Islamic Azad University of Medical Sciences Tabriz Branch (protocol number: IR.IAU.TABRIZ.REC.1401.198) and registered with the local World Health Organization Registry Network (IRCT20220510054805N1).
References
- Atieh MA, AlAli F, Alsabeeha NH. Outcome of supportive peri-implant therapy on the rates of peri-implant diseases and marginal bone loss: a systematic review and meta-analysis. Quintessence Int 2021; 52(2):122-31. doi: 10.3290/j.qi.a45428 [Crossref] [ Google Scholar]
- Berryman Z, Bridger L, Hussaini HM, Rich AM, Atieh M, Tawse-Smith A. Titanium particles: an emerging risk factor for peri-implant bone loss. Saudi Dent J 2020; 32(6):283-92. doi: 10.1016/j.sdentj.2019.09.008 [Crossref] [ Google Scholar]
- Atieh MA, Shah M, Alsabeeha NH. Etiology of peri-implantitis. Curr Oral Health Rep 2020; 7(3):313-20. doi: 10.1007/s40496-020-00272-4 [Crossref] [ Google Scholar]
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018; 45 Suppl 20:S286-91. doi: 10.1111/jcpe.12957 [Crossref] [ Google Scholar]
- Wada M, Mameno T, Otsuki M, Kani M, Tsujioka Y, Ikebe K. Prevalence and risk indicators for peri-implant diseases: a literature review. Jpn Dent Sci Rev 2021; 57:78-84. doi: 10.1016/j.jdsr.2021.05.002 [Crossref] [ Google Scholar]
- Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function-a multicentre retrospective study. Clin Oral Implants Res 2019; 30(2):111-20. doi: 10.1111/clr.13397 [Crossref] [ Google Scholar]
- Daubert DM, Weinstein BF. Biofilm as a risk factor in implant treatment. Periodontol 2000 2019; 81(1):29-40. doi: 10.1111/prd.12280 [Crossref] [ Google Scholar]
- French D, Grandin HM, Ofec R. Retrospective cohort study of 4,591 dental implants: analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J Periodontol 2019; 90(7):691-700. doi: 10.1002/jper.18-0236 [Crossref] [ Google Scholar]
- Kormas I, Pedercini C, Pedercini A, Raptopoulos M, Alassy H, Wolff LF. Peri-implant diseases: diagnosis, clinical, histological, microbiological characteristics and treatment strategies A narrative review. Antibiotics (Basel) 2020; 9(11):835. doi: 10.3390/antibiotics9110835 [Crossref] [ Google Scholar]
- Renvert S, Hirooka H, Polyzois I, Kelekis-Cholakis A, Wang HL. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants - consensus report of working group 3. Int Dent J 2019; 69(Suppl 2):12-7. doi: 10.1111/idj.12490 [Crossref] [ Google Scholar]
- Al Deeb M, Alsahhaf A, Mubaraki SA, Alhamoudi N, Al-Aali KA, Abduljabbar T. Clinical and microbiological outcomes of photodynamic and systemic antimicrobial therapy in smokers with peri-implant inflammation. Photodiagnosis Photodyn Ther 2020; 29:101587. doi: 10.1016/j.pdpdt.2019.101587 [Crossref] [ Google Scholar]
- Iorio-Siciliano V, Blasi A, Stratul SI, Ramaglia L, Sculean A, Salvi GE. Anti-infective therapy of peri-implant mucositis with adjunctive delivery of a sodium hypochlorite gel: a 6-month randomized triple-blind controlled clinical trial. Clin Oral Investig 2020; 24(6):1971-9. doi: 10.1007/s00784-019-03060-2 [Crossref] [ Google Scholar]
- Mariani GM, Ercoli E, Guzzi N, Bongiovanni L, Bianco L, Romano F. One-year clinical outcomes following non-surgical treatment of peri-implant mucositis with adjunctive diode laser application. Minerva Stomatol 2020; 69(5):269-77. doi: 10.23736/s0026-4970.20.04340-x [Crossref] [ Google Scholar]
- Philip J, Laine ML, Wismeijer D. Adjunctive effect of mouthrinse on treatment of peri-implant mucositis using mechanical debridement: a randomized clinical trial. J Clin Periodontol 2020; 47(7):883-91. doi: 10.1111/jcpe.13295 [Crossref] [ Google Scholar]
- Barootchi S, Ravidà A, Tavelli L, Wang HL. Nonsurgical treatment for peri-implant mucositis: a systematic review and meta-analysis. Int J Oral Implantol (Berl) 2020; 13(2):123-39. [ Google Scholar]
- de Carvalho RD, Casarin RC, de Lima PO, Cogo-Müller K. Statins with potential to control periodontitis: from biological mechanisms to clinical studies. J Oral Biosci 2021; 63(3):232-44. doi: 10.1016/j.job.2021.06.002 [Crossref] [ Google Scholar]
- Di Spirito F, Schiavo L, Pilone V, Lanza A, Sbordone L, D’Ambrosio F. Periodontal and peri-implant diseases and systemically administered statins: a systematic review. Dent J (Basel) 2021; 9(9):100. doi: 10.3390/dj9090100 [Crossref] [ Google Scholar]
- Akram Z, Vohra F, Javed F. Efficacy of statin delivery as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a meta-analysis. J Investig Clin Dent 2018; 9(2):e12304. doi: 10.1111/jicd.12304 [Crossref] [ Google Scholar]
- Pradeep AR, Garg V, Kanoriya D, Singhal S. 12% rosuvastatin versus 12% atorvastatin gel local drug delivery and redelivery in treatment of intrabony defects in chronic periodontitis: a randomized placebo-controlled clinical trial. J Periodontol 2016; 87(7):756-62. doi: 10.1902/jop.2016.150706 [Crossref] [ Google Scholar]
- Bassetti M, Schär D, Wicki B, Eick S, Ramseier CA, Arweiler NB. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 2014; 25(3):279-87. doi: 10.1111/clr.12155 [Crossref] [ Google Scholar]
- Pradeep AR, Kumari M, Rao NS, Martande SS, Naik SB. Clinical efficacy of subgingivally delivered 12% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2013; 84(7):871-9. doi: 10.1902/jop.2012.120393 [Crossref] [ Google Scholar]
- Allen LV Jr, Ansel HC. Parenterals. In: Howes S, ed. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 508-77.
- Lowe R, Taylor K. Parenteral drug delivery. In: Aulton ME, Taylor K, eds. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines. 4th ed. Elsevier; 2013. p. 623-37.
- Nagarsenkar MS, Dhawan VV. Parenteral preparations. In: Adejare A, ed. Remington: The Science and Practice of Pharmacy. 23rd ed. Academic Press; 2021. p. 577-603. 10.1016/b978-0-12-820007-0.00029-5.
- Emani S, Gunjiganur GV, Mehta DS. Determination of the antibacterial activity of simvastatin against periodontal pathogens, Porphyromonasgingivalis and Aggregatibacteractinomycetemcomitans: an in vitro study. Contemp Clin Dent 2014; 5(3):377-82. doi: 10.4103/0976-237x.137959 [Crossref] [ Google Scholar]
- Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. Statins in nonsurgical and surgical periodontal therapy A systematic review and meta-analysis of preclinical in vivo trials. J Periodontal Res 2018; 53(3):267-87. doi: 10.1111/jre.12514 [Crossref] [ Google Scholar]
- Bertl K, Parllaku A, Pandis N, Buhlin K, Klinge B, Stavropoulos A. The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-a systematic review and meta-analysis. J Dent 2017; 67:18-28. doi: 10.1016/j.jdent.2017.08.011 [Crossref] [ Google Scholar]
- Rosenberg DR, Andrade CX, Chaparro AP, Inostroza CM, Ramirez V, Violant D. Short-term effects of 2% atorvastatin dentifrice as an adjunct to periodontal therapy: a randomized double-masked clinical trial. J Periodontol 2015; 86(5):623-30. doi: 10.1902/jop.2015.140503 [Crossref] [ Google Scholar]
- Saxlin T, Suominen-Taipale L, Knuuttila M, Alha P, Ylöstalo P. Dual effect of statin medication on the periodontium. J Clin Periodontol 2009; 36(12):997-1003. doi: 10.1111/j.1600-051X.2009.01484.x [Crossref] [ Google Scholar]
- Kumari M, Martande SS, Pradeep AR, Naik SB. Efficacy of subgingivally delivered 12% atorvastatin in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. J Periodontol 2016; 87(11):1278-85. doi: 10.1902/jop.2016.130227 [Crossref] [ Google Scholar]
- Lindy O, Suomalainen K, Mäkelä M, Lindy S. Statin use is associated with fewer periodontal lesions: a retrospective study. BMC Oral Health 2008; 8:16. doi: 10.1186/1472-6831-8-16 [Crossref] [ Google Scholar]
- Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol 2005; 76(11):1861-70. doi: 10.1902/jop.2005.76.11.1861 [Crossref] [ Google Scholar]
- Greenstein G. Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results. J Periodontol 2006; 77(4):565-78. doi: 10.1902/jop.2006.050140 [Crossref] [ Google Scholar]
- Zhao BJ, Liu YH. Simvastatin induces the osteogenic differentiation of human periodontal ligament stem cells. Fundam Clin Pharmacol 2014; 28(5):583-92. doi: 10.1111/fcp.12050 [Crossref] [ Google Scholar]
- Saewong S, Thammasitboon K, Wattanaroonwong N. Simvastatin induces apoptosis and disruption of the actin cytoskeleton in human dental pulp cells and periodontal ligament fibroblasts. Arch Oral Biol 2013; 58(8):964-74. doi: 10.1016/j.archoralbio.2013.03.002 [Crossref] [ Google Scholar]
- Khurana S, Gupta S, Bhalla H, Nandwani S, Gupta V. Comparison of anti-inflammatory effect of atorvastatin with rosuvastatin in patients of acute coronary syndrome. J Pharmacol Pharmacother 2015; 6(3):130-5. doi: 10.4103/0976-500x.162011 [Crossref] [ Google Scholar]
- Ma Q, Zhou Y, Zhai G, Gao F, Zhang L, Wang J. Meta-analysis comparing rosuvastatin and atorvastatin in reducing concentration of C-reactive protein in patients with hyperlipidemia. Angiology 2016; 67(6):526-35. doi: 10.1177/0003319715599863 [Crossref] [ Google Scholar]
- Morris MS, Lee Y, Lavin MT, Giannini PJ, Schmid MJ, Marx DB. Injectable simvastatin in periodontal defects and alveolar ridges: pilot studies. J Periodontol 2008; 79(8):1465-73. doi: 10.1902/jop.2008.070659 [Crossref] [ Google Scholar]
- Fajardo ME, Rocha ML, Sánchez-Marin FJ, Espinosa-Chávez EJ. Effect of atorvastatin on chronic periodontitis: a randomized pilot study. J Clin Periodontol 2010; 37(11):1016-22. doi: 10.1111/j.1600-051X.2010.01619.x [Crossref] [ Google Scholar]
- Goes P, Lima AP, Melo IM, Rêgo RO, Lima V. Effect of atorvastatin in radiographic density on alveolar bone loss in Wistar rats. Braz Dent J 2010; 21(3):193-8. doi: 10.1590/s0103-64402010000300003 [Crossref] [ Google Scholar]