J Adv Periodontol Implant Dent. 16(2):94-102.
doi: 10.34172/japid.2024.021
Research Article
Efficacy of injectable platelet-rich fibrin (i-PRF) as a novel vehicle for local drug delivery in non-surgical periodontal pocket therapy: A randomized controlled clinical trial
Murugan Thamaraiselvan Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Nadathur Doraisamy Jayakumar Data curation, Formal analysis, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing, 1
Author information:
1Department of Periodontics, Saveetha Dental College & Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamilnadu, India
Abstract
Background.
The vehicle in a local drug delivery (LDD) system plays a vital role in delivering the active drug component at the diseased site. Liquid/injectable platelet-rich fibrin (i-PRF), an autologous fibrin matrix, might be used as a vehicle to enmesh drugs and deliver locally at the periodontally diseased sites. This study evaluated the efficacy of the drug (ciprofloxacin [Cip])-loaded i-PRF as a LDD system adjunct to subgingival debridement in subjects with periodontal pockets.
Methods.
In a parallel design study, 79 periodontally diseased pocket sites were randomized to 3 groups: group 1 (n=25), scaling and root planing (SRP)+i-PRF+Cip; group 2 (n=25), SRP+i-PRF; group 3 (n=25), SRP without any adjunctive intervention. Clinical parameters (probing depth [PD], clinical attachment level [CAL], gingival index [GI], plaque index [PI]) and microbial quantification (relative quantification of levels of Aggregatibacter actinomycetemcomitans) were assessed from baseline to 6th and 12th weeks of follow-up.
Results.
All the treatment groups showed significant improvements in the clinical and microbial parameters assessed. Group 1 showed significantly higher PD and GI reduction with CAL gain and decreased in relative levels of A. actinomycetemcomitans in the 12th week, followed by group 2 compared to group 3.
Conclusion.
Thus, within the limits of this study, it can be concluded that i-PRF could be considered a potential LDD vehicle for the delivery of ciprofloxacin in periodontal pocket therapy.
Keywords: Drug carriers, Platelet-rich fibrin, Periodontal disease
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The authors declare that the study was self-funded by Thamaraiselvan Murugan.
Introduction
Periodontitis is an inflammatory disease of the supporting structures of the teeth with a microbial origin and modified by multiple host and environmental factors.1,2 Polymicrobial biofilm is a prerequisite for the initiation of the disease, resulting in a host‒microbial interaction, leading to the destruction of host tissues, including alveolar bone, cementum, and periodontal ligament.3 Although conventional mechanical periodontal therapy (scaling and root planing [SRP])4 targets eliminating these microbial biofilms with the reversal of the inflammatory process, clinical scenarios like deep pockets around inaccessible/difficult-to-access areas like furcation might pose limitations in the complete removal of the plaque biofilm, resulting in residual microbiota within the pocket, favoring further progression of periodontal disease.5,6 Additionally, the inherent nature of certain pathogens like Porphyromonas gingivalis to infiltrate the connective tissue of the periodontal pockets can potentially repopulate the debrided pocket, resulting in relapse.7 Furthermore, host factors like excessive, uncontrolled, or defective immune responses seen in diabetics and smokers might result in continuous tissue destruction with impaired tissue repair.8,9 All these situations demand an additional intervention to the conventional mechanical periodontal therapy, like systemic agents including antibiotics or host-modulating drugs that target the residual periodontal pathogens or the host immune and inflammatory responses, respectively, together constituiting adjunct periodontal therapies.10 However, apart from their promising improved clinical benefits compared to conventional mechanical therapy,11,12 they result in more frequent adverse events like antibiotic resistance and other systemic side effects that have questioned the risk-benefit ratio.13,14
In periodontal therapy, local drug delivery (LDD) has been in practice for the past three decades in treating localized pockets, showing improved clinical and microbiological parameters comparable to adjunctive systemic antibiotic therapy.9,10 Additionally, it has the benefits of a low dose of drug use sufficient to attain the required minimal inhibitory concentrations (MIC) in periodontal tissue, absence of systemic effects, sustained release of drugs, absence of resistance formation, etc.14 Although the current LDD systems like fibers, chips, and gels have overcome the shortcomings of systemic therapy, they still pose some challenges like synthetic or exogenous origin, time consumed for the placement (fibers), a local inflammatory reaction to the degraded products, discomfort to the patient, chances of displacement/dislodgement from the site, need to be removed after the therapy, high cost and some reports (unclear data) about transient antimicrobial resistance.15-17 Newer systems like in-situ gel formulations (hydrogels, polysaccharides, and polymers) with unique properties of sol-to-gel conversion, when influenced by biological stimuli (pH, temperature, and ion exchange) are reported to be more biocompatible and easy to handle.17,18 However, challenges like the complexity of the system, responsiveness to bio-stimulus, reproducibility in terms of performance, lack of tissue integration, and high cost prevent its subsequent translation to clinical use.19 Apart from these, the non-degradability of most synthetic in situ gel formulations also poses an additional challenge to the above list.19 Hence, it is recommended to switch to in situ gel formulations of natural polymer origin that are non-toxic and biodegradable. Thus, the search for an ideal vehicle in periodontal therapy is still continuing.
In this context, platelet-rich fibrin (PRF), a second-generation platelet concentrate,20,21 is widely used in oral, dental, and periodontal regenerative applications. It has a fibrin matrix framework that enmeshes platelets, leukocytes, growth factors (GFs), and other cytokines.22 A modification of the centrifugation protocol results in a liquid form of PRF (liquid PRF) that slowly polymerizes to become a fibrin clot (gel) after approximately 3 minutes, which can be injected into tissues.23 In both forms of PRF, the fibrin matrix plays a crucial role by mimicking a 3D scaffold loaded with growth factors, which gradually degrades, resulting in sustained release of the content into the regeneration area.24-29 Liquid/i-PRF, apart from being syringeable and injectable, is also an autologous product that shows good tissue integration and can facilitate the localization of the PRF clot with the required site. Considering the above benefits (sol-to-gel transition, injectable, autologous, bioadhesive [tissue integration]) and the 3D architecture of the iPRF, we thought that it could be used as a vehicle to enmesh drugs and deliver locally at the periodontally diseased sites. Our recent in vitro study using i-PRF as a vehicle for local delivery of three drugs (ciprofloxacin, curcumin, and tannic acid) showed a sustained release pattern of all three drugs with only 59%, 64%, and 20% of the loaded drug released at the end of the observation period (14 days). These study results lead us to continue this human clinical trial. Thus, this study evaluated the efficacy of the drug (ciprofloxacin [Cip])-loaded i-PRF as a LDD system adjunct to subgingival debridement in subjects with periodontal pockets.
Methods
The present single-centered, randomized, controlled parallel-design study was carried out in the Department of Periodontics at a university dental hospital setting adhering to CONSORT guidelines (Figure 1). After the study design was approved by the institutional Ethics Committee and institutional Review Board (1HEC Ref No: IHEC/SDC/PERIO-1802/22/573), it was registered in the clinical trial registry (http://ctri.nic.in) (CTRI/2023/01/048659 [Registered on: 02/01/2023]). The study protocol also follows Helsinki’s declaration for human trials as revised in 2008.
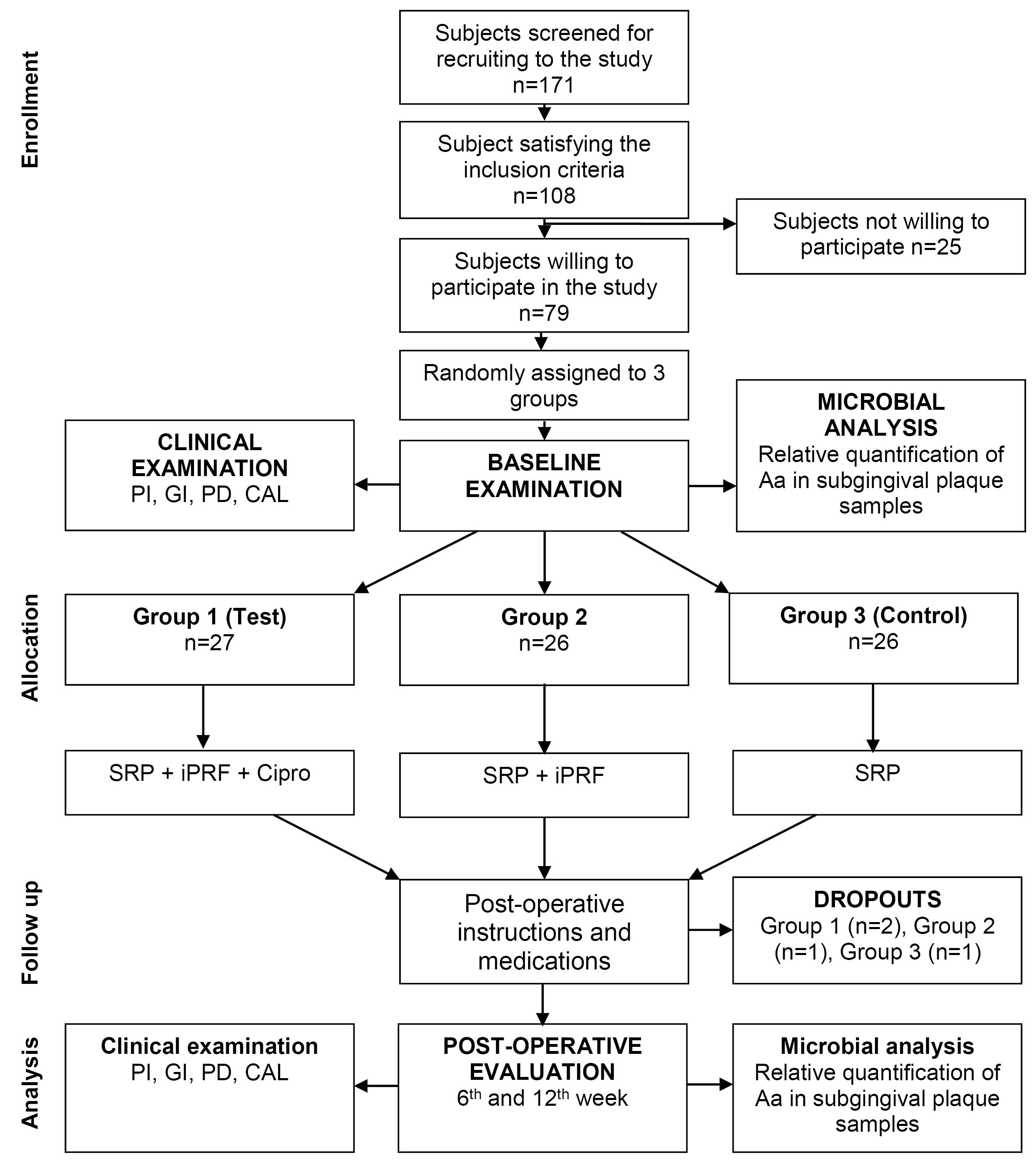
Figure 1.
Flowchart showing the study design and protocol (CONSORT Diagram)
.
Flowchart showing the study design and protocol (CONSORT Diagram)
The sample size for the clinical trial was calculated using the formula reported by Chow et al.30 The estimation indicated that at least a sample size of 12 is required to gain 80% power and show a difference in mean probing depth (PD) between 3 groups at the end of 12 weeks of evaluation. A final sample of n = 25 in each group was decided to compensate for the dropouts during the study.
The study population was selected from the subjects visiting the outpatient section of the Department of Periodontics at Saveetha Dental College and Hospitals between January 2023 and March 2023 based on the following inclusion and exclusion criteria.
Inclusion criteria
-
The age group of 30‒60 years
-
Subjects diagnosed with Stage II Grade B periodontitis based on the 2018 classification31
-
Subjects with at least 20 teeth at the time of initial examination
-
Subjects with periodontal condition showing PD of ≥ 5 mm in at least two sites
-
Subjects with periodontal conditions showing clinical attachment loss (CAL) of ≥ 2 mm in at least two sites
Exclusion criteria
-
Pregnant and lactating women
-
Use of immunosuppressive medications, consumption of antibiotics, and any antioxidants and anti‐inflammatory agents in the last three months
-
A history of periodontal therapy in the preceding one year
-
Subjects with hemoglobin levels < 11 mg/dL
-
Subject participating in any other clinical trials
A total of 108 subjects meeting the study criteria were briefed about the study protocol and asked for written consent. Of these, 79 subjects gave written consent to participate in the study, each with one experimental site contributing to 79 sites.
Baseline examination
For all the enrolled participants, baseline plaque samples were collected according to the standard protocol at the experimental site, as follows, by a single blinded examiner. After supragingival plaque removal using a curette, adequate isolation was achieved around the experimental sites using cotton rolls.
-
Plaque samples were collected from the subgingival environment using #25 sterile paper points placed into the experimental pocket site to reach the bottom and left in place for 15 seconds. Then, the paper point was transferred to a vial containing 1 mL of viability medium Gothenburg anaerobic (VMGA) transport medium and sent to the laboratory under anaerobic conditions immediately. After vortexing for 60 seconds, the samples were used for the polymerase chain reaction (PCR) analysis.
This was followed by clinical data collection using a Williams periodontal probe as follows:
-
Plaque index (PI) based on Silness and Löe (1964)
-
Gingival index (GI) based on Silness and Löe (1963)
-
PD measured from the marginal gingiva to the base of the pocket
-
Clinical attachment level (CAL) measured from the cementoenamel junction to the base of the pocket
All the participants underwent phase 1 periodontal therapy, Including complete ultrasonic scaling with root planing at localized periodontal pocket sites with Gracey curettes by a single operator. Following this, periodontal pockets were irrigated with copious saline to flush the disrupted biofilm and calculus out of the pocket environment.
These subjects were then randomly assigned to one of the three groups using computer-generated numbers using the SAS®. The allocation concealment for the groups was done using sequentially numbered, sealed, opaque envelopes. All the randomization and allocation concealment were done by a separate examiner (JK) other than the principal investigator.
-
Group 1 – Drug-loaded injectable platelet-rich fibrin (i-PRF): After SRP, this group was treated with the delivery of ciprofloxacin-loaded i-PRF into the periodontal pocket and gingival tissue adjacent to the pocket wall. The i-PRF was applied only once and was not repeated during the study period.
-
Group 2 – Drug-free i-PRF: After SRP, this group was treated with the delivery of drug-free i-PRF into the periodontal pocket and gingival tissue adjacent to the pocket. The i-PRF was applied only once and was not repeated during the study period.
-
Group 3 – SRP: This group was treated only with SRP without any adjunct therapy.
Once the allocation was done, for the participants in group 3, this was the only therapy rendered. In contrast, for the participants in groups 1 and 2, i-PRF with and without ciprofloxacin was applied as an adjunct to SRP local delivery, respectively. The i-PRF and i-PRF loaded with ciprofloxacin were prepared as follows.
Collection of i-PRF
The iPRF was prepared by the same operator according to the protocol developed by Miron and Choukron in 2017.32 It involves collecting 10 mL of intravenous blood from the participant using venipuncture of antecubital vein under sterile conditions. The collected blood is transferred to a plain sterile test tube without anticoagulant and immediately subjected to centrifugation at 70-g force at 700 rpm for 3 minutes. After centrifugation, the blood separates into two parts: the bottom layer consists of a red blood cell compartment, and the top layer is PRF plasma, which is still in liquid consistency. The top PRF layer is aspirated into a 2-mL syringe and maintained in liquid consistency for about 3‒5 minutes until it clots by slow polymerization of fibrin formation.
Preparation of the drug solution
The concentration of the ciprofloxacin drug to be loaded in iPRF was decided based on our team’s earlier in vitro cytocompatibility and drug release kinetic study (unpublished data). According to the data obtained, 1 mg/mL of the drug concentration was found to be biocompatible with maximum efficacy and showed a sustained release of 59% of loaded drug at the end of 14-day observation. Analytical grade ciprofloxacin hydrochloride was obtained from Sigma-Aldrich Corp. (Milwaukee, Wis., USA). One milligram of the drug was weighed and mixed with 100 µL of deionized water and shaken for 30 seconds to make the drug completely soluble, which was done just before the blood collection from the participants.
Preparation of the drug-loaded i-PRF
Then, 900 of the obtained i-PRF was dispensed in a vial containing a 1-mg/100 µL solution of ciprofloxacin and shaken gently for 10 seconds to obtain a homogenous mix with a final concentration of 1 mg/mL (Figure 2).

Figure 2.
i-PRF mixed with ciprofloxacin drug in liquid consistency
.
i-PRF mixed with ciprofloxacin drug in liquid consistency
Local delivery of ciprofloxacin-loaded i-PRF
This mixture was further immediately loaded in a 1-mL insulin syringe (Figure 3) and injected into the periodontal pocket until it filled and overflowed followed by injecting into the tissues adjacent to the periodontal pocket before it became a gel in the participants of group 1 (Figure 4). In group 2 participants, plain iPRF was delivered at the experimental sites as explained above and allowed to gel. Postoperative instructions were given to all the study participants. There was no prescription for mouthwash or medications for any of the subjects, and they were asked to report after 6 and 12 weeks for follow-up.

Figure 3.
i-PRF mixed with ciprofloxacin loaded in an insulin syringe
.
i-PRF mixed with ciprofloxacin loaded in an insulin syringe
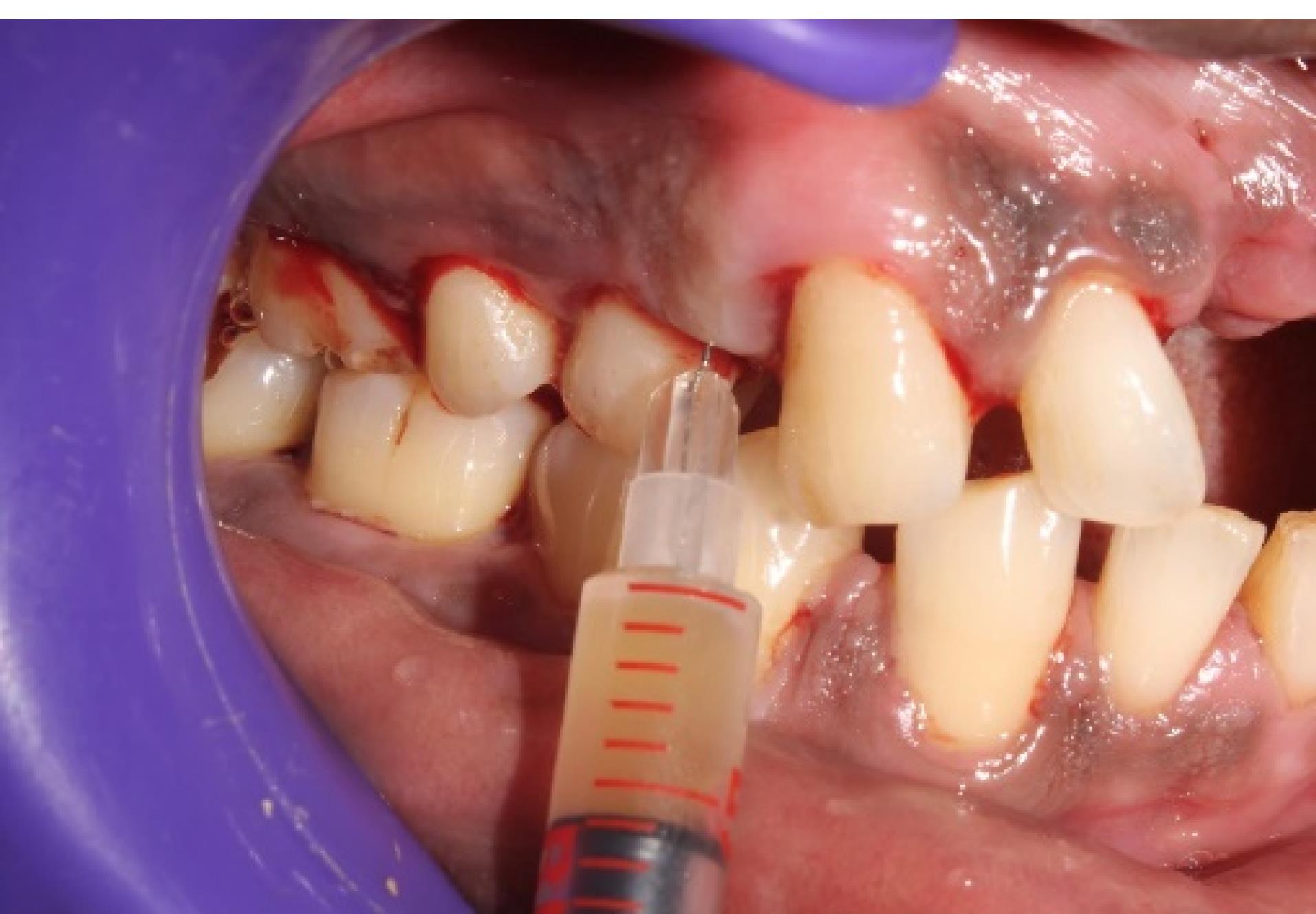
Figure 4.
i-PRF mixed with ciprofloxacin injected into the periodontal pocket (group 1)
.
i-PRF mixed with ciprofloxacin injected into the periodontal pocket (group 1)
Follow-up examinations
All the clinical measurements and plaque sample collection procedures were performed by the same calibrated examiner (SS) with an intra-observer (first and second readings) reliability expressed as a weighted Kappa coefficient with a 95% confidence interval. The operator was blinded throughout the study. The plaque sample collection and clinical examination (PD, CAL, GI, and PI) were performed at the 6th- and 12th-week follow-ups.
Microbial analysis
From the plaque samples collected from the subgingival area, relative quantification of the level of Aggregatibacter actinomycetemcomitans in the total level of bacterial cells was assessed using TaqMan real-time polymerase chain reaction (RT-PCR). The DNA extraction and isolation were performed using a standardized method described in the laboratory manual for molecular cloning from the plaque biofilm samples.33 The A. actinomycetemcomitans (ATCC 29523) bacterial DNA identification was performed using the bacterial DNA template and bacteria-specific primers and probes that were processed as recommended by the manufacturer with the following amplification protocols (50 °C for 2 minutes, 95 °C for 10 minutes, and then 60 cycles of 15 seconds at 95 °C and 1 minute at 58 °C). The sequence and PCR procedures have been previously described in detail by Kato et al.34 For the relative quantification, the copy numbers of pathogenic bacterial genes were standardized to the copy number of the 16S rRNA genes by using the simplified comparative threshold cycle (∆Ct) method reported by Yoshida et al.35
Primary and secondary outcomes
The primary outcomes are the changes in clinical parameters, such as a reduction in PD, which was evaluated in the 12th week. The secondary outcomes are changes in PI, GI, gain in CAL, and relative quantity of A. actinomycetemcomitans.
Statistical analysis
The results of the clinical parameters are expressed as mean ± standard deviation. All the parameters were compared within groups at different time intervals using one-way ANOVA, while inter-group comparison was made using ANOVA followed by post hoc analysis by the Bonferroni test. The microbial analysis reported the relative proportion of A. actinomycetemcomitans bacterial cell counts at different time intervals as mean counts (log10) ± SD. Comparisons between groups at different time intervals were made using one-way ANOVA, while comparisons between groups were made using ANOVA.
Results
All the recruited subjects underwent the intervention (group 1 [n = 27], group 2 [n = 26], group 3 [n = 26]). During the follow-up, there were two dropouts from group 1 and one from each of the groups 2 and 3, resulting in 25 final samples in each group to be considered for final statistical analysis. No adverse reactions were observed during the study period for any of the interventions, and none of the participants reported any discomfort with the treatment protocol (Figures 5 and 6). The results are shown as mean ± standard deviation (SD) for clinical parameters and mean counts (log10) ± SD for microbiological parameters in Table 1 to Table 5.
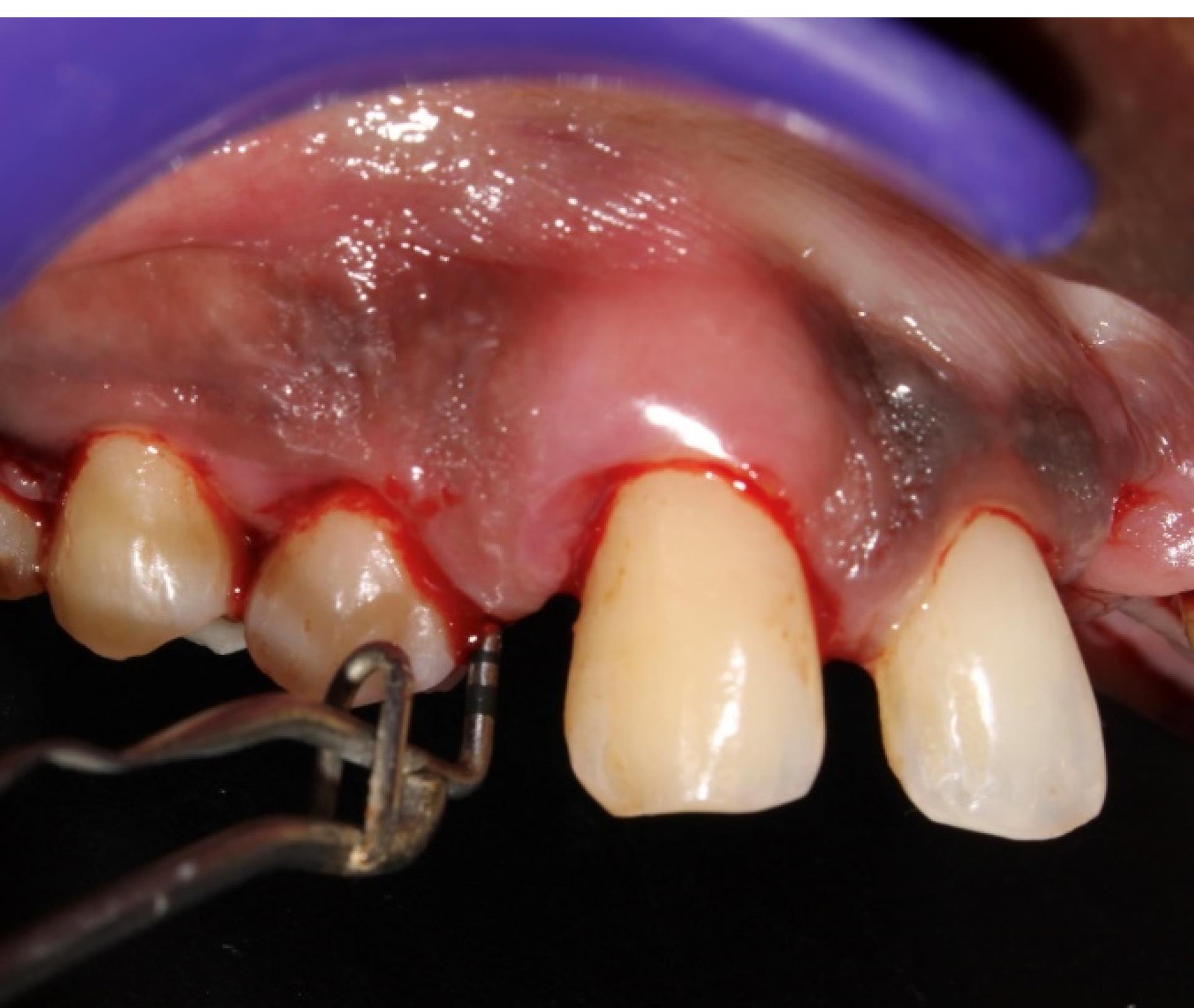
Figure 5.
Preoperative picture (Baseline) showing PD in one of the experimental sites (#5) (group 1)
.
Preoperative picture (Baseline) showing PD in one of the experimental sites (#5) (group 1)
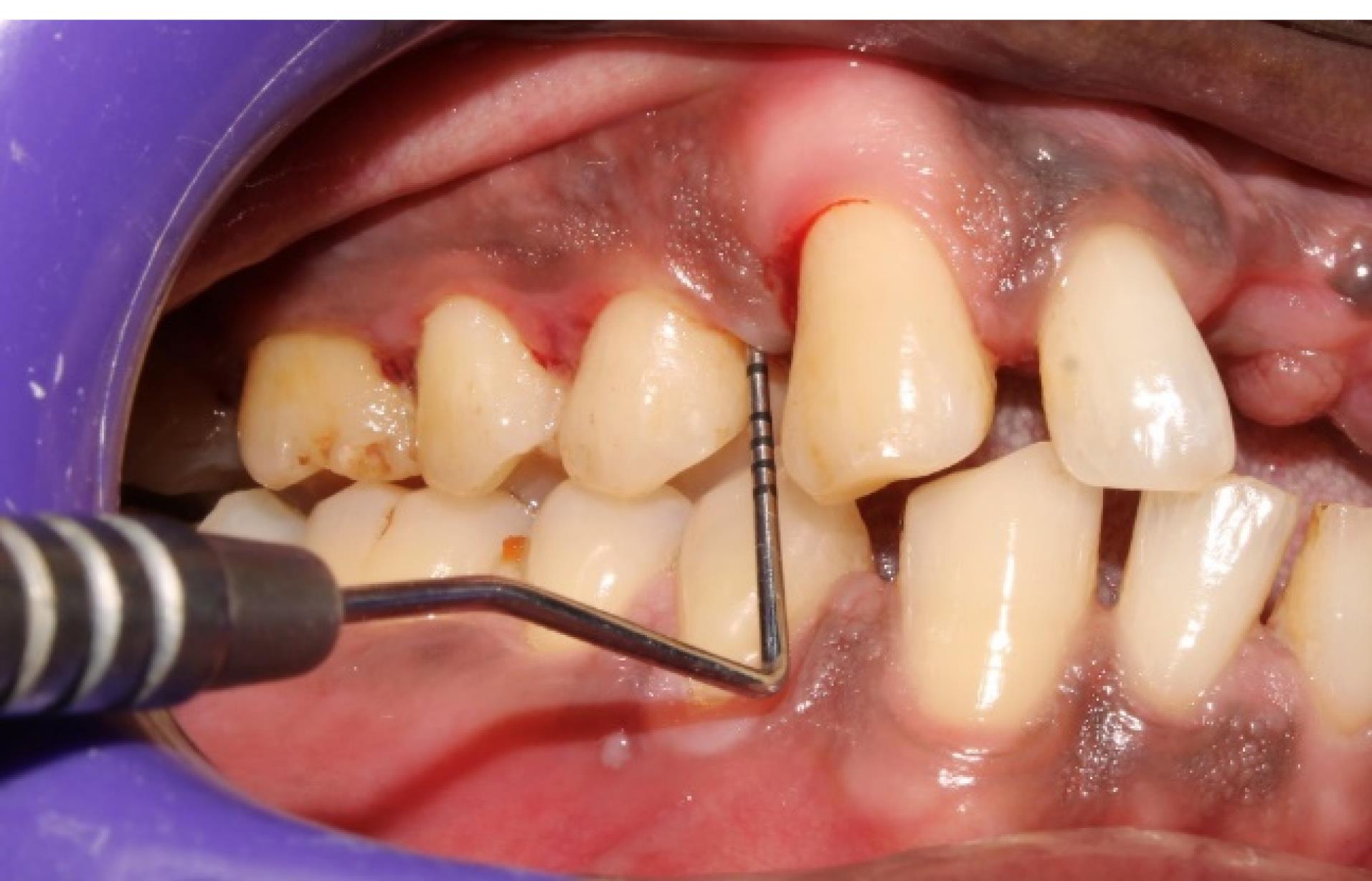
Figure 6.
Postoperative picture (12th week) showing reduced PD in the experimental site (#5) shown in Figure 5 (group 1)
.
Postoperative picture (12th week) showing reduced PD in the experimental site (#5) shown in Figure 5 (group 1)
Table 1.
Comparison of probing depths (PD) within and between groups
|
PD (mm)
|
Group 1 (n=25)
|
Group 2 (n=25)
|
Group 3 (n=25)
|
P
value
|
| Baseline |
5.66 ± 0.63 |
5.45 ± 0.65 |
5.83 ± 0.76 |
0.893 |
| 6th week |
3.91 ± 0.58‡§ |
4.41 ± 0.65§ |
4.87 ± 0.79§ |
0.413 |
| 12th week |
3.70 ± 0.46‡§ |
4.33 ± 0.56†§ |
4.79 ± 0.72§ |
0.002‖ |
PD: probing depth; group 1: drug-loaded i-PRF (injectable platelet-rich fibrin); group 2: drug-free i-PRF; group 3: SRP (scaling and root planing).
†Statistically significant (P < 0.05) compared to group 3.
‡Statistically highly significant (P < 0.01) compared to group 3.
§Statistically significant (P < 0.05) compared to baseline.
‖Statistically significant (P < 0.05) when compared between groups 1 and 2.
Table 2.
Comparison of clinical attachment level (CAL) within and between groups
|
CAL (mm)
|
Group 1 (n=25)
|
Group 2 (n=25)
|
Group 3 (n=25)
|
P
value
|
| Baseline |
4.54 ± 0.58 |
4.37 ± 0.71 |
4.66 ± 0.70 |
1.000 |
| 6th week |
2.33 ± 0.56‡§ |
2.91 ± 0.77‡§ |
3.91 ± 0.65 § |
0.117 |
| 12th week |
2.33 ± 0.56‡§ |
2.91 ± 0.77†§ |
4.00 ± 0.65§ |
0.011‖ |
CAL: clinical attachment level; group 1: drug-loaded i-PRF (injectable platelet-rich fibrin); group 2: drug-free i-PRF; group 3: SRP (scaling and root planing).
†Statistically significant (P < 0.05) compared to group 3.
‡Statistically highly significant (P < 0.01) compared to group 3.
§Statistically significant (P < 0.05) compared to baseline.
‖Statistically significant (P < 0.05) when compared between groups 1 and 2.
Table 3.
Comparison of gingival index (GI) within and between groups
|
GI (%)
|
Group 1 (n=25)
|
Group 2 (n=25)
|
Group 3 (n=25)
|
P
value
|
| Baseline |
1.66 ± 0.48 |
1.87 ± 0.33 |
1.66 ± 0.48 |
0.314 |
| 6th week |
0.85 ± 0.15‡§ |
0.85 ± 0.16‡§ |
1.15 ± 0.12§ |
1.000 |
| 12th week |
0.91 ± 0.22‡§ |
0.88 ± 0.14‡§ |
1.15 ± 0.12§ |
1.000 |
GI: gingival index; group 1: drug-loaded i-PRF (injectable platelet-rich fibrin); group 2: drug-free i-PRF; group 3: SRP (scaling and root planing).
†Statistically significant (P < 0.05) compared to group 3.
‡Statistically highly significant (P < 0.01) compared to group 3.
§Statistically significant (P < 0.05) compared to baseline.
‖Statistically significant (P < 0.05) when compared between groups 1 and 2.
Table 4.
Comparison of plaque index (PI) within and between groups
|
PI (%)
|
Group 1 (n=25)
|
Group 2 (n=25)
|
Group 3 (n=25)
|
P
value
|
| Baseline |
1.45 ± 0.50 |
1.57 ± 0.49 |
1.50 ± 0.51 |
1.000 |
| 6th week |
0.74 ± 0.19§ |
0.65 ± 0.16§ |
0.64 ± 0.10§ |
0.144 |
| 12th week |
0.68 ± 0.09§ |
0.65 ± 0.08†§ |
0.69 ± 0.10§ |
1.000 |
PI: plaque index;group 1: drug-loaded iPRF (injectable platelet-rich fibrin); group 2: drug-free i-PRF; group 3: SRP (scaling and root planing); BL, baseline.
†Statistically significant (P < 0.05) compared to group 3.
‡Statistically highly significant (P < 0.01) compared to group 3.
§Statistically significant (P < 0.05) compared to baseline.
‖Statistically significant (P < 0.05) when compared between groups 1 and 2.
Table 5.
Comparison of Aggregatibacter actinomycetemcomitans levels within and between groups
|
A. actinomycetemcomitans (Mean counts (log10)±SD)
|
Group 1 (n=25)
|
Group 2 (n=25)
|
Group 3 (n=25)
|
P
value
|
| Baseline |
0.97 ± 0.21 |
1.04 ± 0.37 |
1.02 ± 0.31 |
1.000 |
| 6th week |
0.14 ± 0.10†§ |
0.27 ± 0.11†§ |
1.27 ± 0.52 |
0.03 ‖ |
| 12th week |
0.19 ± 0.17†§ |
0.35 ± 0.12†§ |
1.32 ± 0.41 |
0.03 ‖ |
PI: plaque index; group 1: drug-loaded iPRF (injectable platelet-rich fibrin); group 2: drug-free i-PRF; group 3: SRP (scaling and root planing).
†Statistically significant (P < 0.05) compared to group 3.
‡Statistically highly significant (P < 0.01) compared to group 3.
§Statistically significant (P < 0.05) compared to baseline.
‖Statistically significant (P < 0.05) when compared between groups 1 and 2
Discussion
Adjunctive use of LDD to SRP in periodontal pocket therapy has been documented thoroughly in the literature, with most of the evidence supporting its additional beneficial effect.36,37 The current research in LDD is more inclined toward developing an ideal vehicle that facilitates sustained release with high biocompatibility with the host tissue.38 The rationale for using iPRF as an LDD vehicle for pocket therapy in the present study stemmed from its mesh-like fibrin architecture that has shown a sustained release of entrapped growth factors at the periodontal wound sites,39,40 with extensive biological activities and advanced safety margin. To our knowledge, this is the first study in the literature to evaluate iPRF as an LDD system in periodontal pocket therapy.
Our study involved a parallel design protocol appropriate for LDD investigations to eliminate the crossover effect.41 Also, our sample distribution showed no significant difference in baseline clinical parameters between the groups, suggesting the elimination of selection bias. The clinical and microbiological outcomes for the intervention were assessed for three months, which was reported to be sufficient to investigate the effect of the LDD.42,43
All the subjects in our study showed good oral hygiene after being included in the study protocol, which was evident in the significant reduction in the PI score concurrent with earlier studies.44 This might be due to the oral hygiene instruction and reinforcement given after scaling protocol and the recruitment of only the subjects who showed satisfactory oral hygiene.
Similarly, there was a significant improvement in GI irrespective of the intervention made in all the treatment groups.45 The resolution of gingival inflammation should have been mediated by the elimination of subgingival plaque biofilm by SRP and improved oral hygiene by the patient in all the treatment groups.45 However, incorporating i-PRF into SRP in groups 1 and 2 resulted in further improvements in GI, which concurs well with earlier reports.46 This may be attributed to the anti-inflammatory effect of the white blood cells and the cytokine released from it.46 The presence of ciprofloxacin in group 1 did not bring about any further resolution of gingival inflammation compared to group 2, suggesting that most of the gingival inflammatory resolution was mediated by the anti-inflammatory effect of i-PRF rather than ciprofloxacin.
All our interventions resulted in a significant PD reduction, with group 1 showing the maximum reduction, followed by group 2 compared to group 3 at the end of the 12th week. This is concurrent with earlier reports where SRP resulted in a significant reduction in PD, which was mainly attributed to the resolution of periodontal inflammation after the elimination of the plaque biofilm.45 Furthermore, the adjunctive use of i-PRF showed a significant PD reduction, which is supported by previous studies.46 This could be due to the delivery of growth factors and other anti-inflammatory cytokines from the PRF matrix to the periodontal pocket that may reduce inflammation and enhance the regenerative potential at the pocket environment, which could have resulted in PD reduction. The highest PD reduction in group I could be due to the antibacterial effect of ciprofloxacin delivered by the i-PRF vehicle. Since no previous studies are available, a direct comparison of our study outcome was not possible. Nevertheless, our results are consistent with earlier reports on other LDD systems showing a significant PD reduction when LDD was used.47 Furthermore, the ciprofloxacin-loaded group (group 1) outperformed the plain i-PRF LDD group (group 2), indicating a possible role of the drug released from the i-PRF vehicle apart from the i-PRF itself.
Evaluation of CAL showed significant attachment gain in all the treatment groups at the 12th week of observation, consistent with earlier reports46,48 where SRP facilitates attachment gain through the formation of long junctional epithelium.49 Among all the groups, the ciprofloxacin-loaded i-PRF group (group 1) showed a significant maximum attachment gain compared to other groups. A possible explanation for this is the potential of fibrin in i-PRF to adhere to the root surface, which could have facilitated a new attachment. However, this should be confirmed with further histologic investigation. Nevertheless, the role of ciprofloxacin, resulting in maximum attachment gain compared to the plain iPRF group, needs to be evaluated.
Aggregatibacter actinomycetemcomitans is one of the primary periodontal pathogens involved in the pathogenesis of periodontal destruction and pocket formation. Hence, measuring it quantitatively (A. actinomycetemcomitans) could be one of the valuable methods for monitoring periodontal disease status and assessment for treatment outcomes. Also, ciprofloxacin, being a broad-spectrum agent and reported to be active against all strains of A. actinomycetemcomitans, could be a validation for the microbial analysis. All our experimental groups resulted in a decrease in the relative levels of A. actinomycetemcomitans, with group 1 showing the maximum changes, followed by groups 2 and 3. As mentioned earlier, the lack of studies limits the direct comparison of our results. However, this is similar to other reports on LDD with fluoroquinolones, significantly reducing A. actinomycetemcomitans levels.47 Between groups 1 and 2, the former resulted in a significant reduction of A. actinomycetemcomitans than the latter. This tendency ensures the possible role of ciprofloxacin in microbial reduction and, in turn, results in improvements in clinical parameters. A possible explanation for the improved clinical and microbiological parameters for three months (12 weeks) could be by the natural biodegradation of the fibrin network of i-PRF fibrin matrix through enzymatic or hydrolytic tissue reaction that could result in gradual and sustained release of ciprofloxacin in the periodontally infected sites thus ensuring the extended availability of the drug.
Apart from the clinical efficacy observed, there are many advantages of using an i-PRF vehicle, which differs from conventional vehicles (synthetic/natural or exogenous) since it is autologous, easy to handle (can be prepared chairside, with no addition of any chemicals, syringeability), and can be injected directly into the periodontal tissues and pocket. Its slow polymerization to gel allows incorporation of the drug and polymerization within the pocket, resulting in the gel’s better adaptation to periodontal pocket dimensions. Additionally, the adherence and integration of i-PRF to the host tissue, like the root surface and gingival tissue, minimizes the dislodgement of the vehicle, thus ensuring sustained drug delivery and, finally, its economic benefits.
Future research to confirm the sustained release by evaluating the availability of loaded drugs in the oral environment is mandatory to add strength to the current evidence. Also, evaluation of the influence of loaded drugs on the biological nature of the i-PRF vehicle has to be ascertained. Other limitations are the shorter length of follow-up and the absence of a positive control group.
From the above observations, the use of ciprofloxacin-loaded i-PRF as an LDD system resulted in a significant reduction of A. actinomycetemcomitans, which in turn contributed to clinical improvements in terms of PD, GI reduction, and CAL gain. Thus, within the limits of this study, it can be concluded that i-PRF could be considered a potential LDD vehicle for the delivery of ciprofloxacin in periodontal pocket therapy.
Acknowledgments
The authors would like to acknowledge the postgraduate resident, Deepika, for her support in scheduling the patients for treatment and follow-up appointments.
Competing Interests
The authors declare that they have no competing interests.
Consent for Publication
Written consent was obtained from all the study subjects for the use of pictures and data during publication.
Data Availability Statement
Individual deidentified participant data collected (Study protocol, informed consent form) during the study will be shared after deidentification; the data will become available immediately after publication for three months for researchers who provide a methodologically sound proposal; data will be shared on request by mail.
Ethical Approval
The study design was approved by the institutional ethical committee and institutional review board (1HEC Ref No: IHEC/SDC/PERIO-1802/22/573) of Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences.
References
- Krishna R, De Stefano JA. Ultrasonic vs hand instrumentation in periodontal therapy: clinical outcomes. Periodontol 2000 2016; 71(1):113-27. doi: 10.1111/prd.12119 [Crossref] [ Google Scholar]
- Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000 2017; 75(1):7-23. doi: 10.1111/prd.12221 [Crossref] [ Google Scholar]
- Nunn ME. Understanding the etiology of periodontitis: an overview of periodontal risk factors. Periodontol 2000 2003; 32:11-23. doi: 10.1046/j.0906-6713.2002.03202.x [Crossref] [ Google Scholar]
- Ioannou I, Dimitriadis N, Papadimitriou K, Sakellari D, Vouros I, Konstantinidis A. Hand instrumentation versus ultrasonic debridement in the treatment of chronic periodontitis: a randomized clinical and microbiological trial. J Clin Periodontol 2009; 36(2):132-41. doi: 10.1111/j.1600-051X.2008.01347.x [Crossref] [ Google Scholar]
- Zappa UE. Factors determining the outcome of scaling and root planing. Probe 1992; 26(4):152-9. [ Google Scholar]
- Drisko CL, Killoy WJ. Scaling and root planing: removal of calculus and subgingival organisms. Curr Opin Dent 1991; 1(1):74-80. [ Google Scholar]
- Lõivukene K, Pähkla ER, Koppel T, Saag M, Naaber P. The microbiological status of patients with periodontitis in southern Estonia after non-surgical periodontal therapy. Stomatologija 2005; 7(2):45-7. [ Google Scholar]
- Chang J, Meng HW, Lalla E, Lee CT. The impact of smoking on non-surgical periodontal therapy: a systematic review and meta-analysis. J Clin Periodontol 2021; 48(1):60-75. doi: 10.1111/jcpe.13384 [Crossref] [ Google Scholar]
- Kanmaz B, Lappin DF, Nile CJ, Buduneli N. Effects of smoking on non-surgical periodontal therapy in patients with periodontitis stage III or IV, and grade C. J Periodontol 2020; 91(4):442-53. doi: 10.1002/jper.19-0141 [Crossref] [ Google Scholar]
- Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. The adjunctive use of host modulators in non-surgical periodontal therapy A systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol 2020; 47 Suppl 22:199-238. doi: 10.1111/jcpe.13232 [Crossref] [ Google Scholar]
- Pal A, Paul S, Perry R, Puryer J. Is the use of antimicrobial photodynamic therapy or systemic antibiotics more effective in improving periodontal health when used in conjunction with localised non-surgical periodontal therapy? A systematic review. Dent J (Basel) 2019; 7(4):108. doi: 10.3390/dj7040108 [Crossref] [ Google Scholar]
- Keestra JA, Grosjean I, Coucke W, Quirynen M, Teughels W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: a systematic review and meta-analysis. J Periodontal Res 2015; 50(3):294-314. doi: 10.1111/jre.12221 [Crossref] [ Google Scholar]
- American Academy of Periodontology. Statement on local delivery of sustained or controlled release antimicrobials as adjunctive therapy in the treatment of periodontitis. J Periodontol 2006; 77(8):1458. doi: 10.1902/jop.2006.068001 [Crossref] [ Google Scholar]
- Ramanauskaite E, Machiulskiene V. Antiseptics as adjuncts to scaling and root planing in the treatment of periodontitis: a systematic literature review. BMC Oral Health 2020; 20(1):143. doi: 10.1186/s12903-020-01127-1 [Crossref] [ Google Scholar]
- Quirynen M, Teughels W, van Steenberghe D. Microbial shifts after subgingival debridement and formation of bacterial resistance when combined with local or systemic antimicrobials. Oral Dis 2003; 9 Suppl 1:30-7. doi: 10.1034/j.1601-0825.9.s1.6.x [Crossref] [ Google Scholar]
- Costa JV, Portugal J, Neves CB, Bettencourt AF. Should local drug delivery systems be used in dentistry?. Drug Deliv Transl Res 2022; 12(6):1395-407. doi: 10.1007/s13346-021-01053-x [Crossref] [ Google Scholar]
- Zhang Y, Jiang R, Lei L, Yang Y, Hu T. Drug delivery systems for oral disease applications. J Appl Oral Sci 2022; 30:e20210349. doi: 10.1590/1678-7757-2021-0349 [Crossref] [ Google Scholar]
- Batool F, Agossa K, Lizambard M, Petit C, Bugueno IM, Delcourt-Debruyne E. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treatment: proof of concept study in vivo. Int J Pharm 2019; 569:118564. doi: 10.1016/j.ijpharm.2019.118564 [Crossref] [ Google Scholar]
- Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv 2012; 9(4):383-402. doi: 10.1517/17425247.2012.665367 [Crossref] [ Google Scholar]
- Miron RJ, Choukroun J. Future research with platelet-rich fibrin. In: Choukroun J, Miron RJ, eds. Platelet-Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications. Oxford: Wiley-Blackwell; 2017. p. 251-61. 10.1002/9781119406792.ch15.
- Choukroun J, Simonpieri A, Girard MO, Fioretti F, Dohan S, Dohan D. Platelet-rich fibrin (PRF): a new healing biomaterial. Implantodontie 2004; 4(13):229-35. doi: 10.1016/j.implan.2004.07.002 [Crossref] [ Google Scholar]
- Lee JW, Kim SG, Kim JY, Lee YC, Choi JY, Dragos R. Restoration of a peri-implant defect by platelet-rich fibrin. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(4):459-63. doi: 10.1016/j.tripleo.2011.03.043 [Crossref] [ Google Scholar]
- Varela HA, Souza JC, Nascimento RM, Araújo RF Jr, Vasconcelos RC, Cavalcante RS. Injectable platelet-rich fibrin: cell content, morphological, and protein characterization. Clin Oral Investig 2019; 23(3):1309-18. doi: 10.1007/s00784-018-2555-2 [Crossref] [ Google Scholar]
- Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 2016; 20(9):2353-60. doi: 10.1007/s00784-016-1719-1 [Crossref] [ Google Scholar]
- Nagaraja S, Mathew S, Abraham A, Ramesh P, Chandanala S. Evaluation of vascular endothelial growth factor - a release from platelet-rich fibrin, platelet-rich fibrin matrix, and dental pulp at different time intervals. J Conserv Dent 2020; 23(4):359-63. doi: 10.4103/jcd.jcd_465_19 [Crossref] [ Google Scholar]
- Thamaraiselvan M, Elavarasu S, Thangakumaran S, Gadagi JS, Arthie T. Comparative clinical evaluation of coronally advanced flap with or without platelet-rich fibrin membrane in the treatment of isolated gingival recession. J Indian Soc Periodontol 2015; 19(1):66-71. doi: 10.4103/0972-124x.145790 [Crossref] [ Google Scholar]
- Panda S, Satpathy A, Chandra Das A, Kumar M, Mishra L, Gupta S. Additive effect of platelet-rich fibrin with coronally advanced flap procedure in root coverage of Miller’s class I and II recession defects-a PRISMA compliant systematic review and meta-analysis. Materials (Basel) 2020; 13(19):4314. doi: 10.3390/ma13194314 [Crossref] [ Google Scholar]
- Bai MY, Wang CW, Wang JY, Lin MF, Chan WP. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo) 2017; 72(2):116-24. doi: 10.6061/clinics/2017(02)09 [Crossref] [ Google Scholar]
- Faour NH, Dayoub S, Hajeer MY. Evaluation of the hyaluronic acid versus the injectable platelet-rich fibrin in the management of the thin gingival phenotype: a split-mouth randomized controlled clinical trial. Cureus 2022; 14(5):e25104. doi: 10.7759/cureus.25104 [Crossref] [ Google Scholar]
- Chow SC, Wang H, Shao J. Sample Size Calculations in Clinical Research. CRC Press; 2003. p. 352.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89 Suppl 1:S173-82. doi: 10.1002/jper.17-0721 [Crossref] [ Google Scholar]
- Karde PA, Sethi KS, Mahale SA, Khedkar SU, Patil AG, Joshi CP. Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: an in vitro study. J Indian Soc Periodontol 2017; 21(2):97-101. doi: 10.4103/jisp.jisp_201_17 [Crossref] [ Google Scholar]
- Bain MJ, Strahan JD. The effect of a 1% chlorhexidine gel in the initial therapy of chronic periodontal disease. J Periodontol 1978; 49(9):469-74. doi: 10.1902/jop.1978.49.9.469 [Crossref] [ Google Scholar]
- Kato H, Yoshida A, Awano S, Ansai T, Takehara T. Quantitative detection of volatile sulfur compound- producing microorganisms in oral specimens using real-time PCR. Oral Dis 2005; 11 Suppl 1:67-71. doi: 10.1111/j.1601-0825.2005.01096.x [Crossref] [ Google Scholar]
- Yoshida A, Suzuki N, Nakano Y, Oho T, Kawada M, Koga T. Development of a 5’ fluorogenic nuclease-based real-time PCR assay for quantitative detection of Actinobacillusactinomycetemcomitans and Porphyromonasgingivalis. J Clin Microbiol 2003; 41(2):863-6. doi: 10.1128/jcm.41.2.863-866.2003 [Crossref] [ Google Scholar]
- Timmerman MF, van der Weijden GA, van Steenbergen TJ, Mantel MS, de Graaff J, van der Velden U. Evaluation of the long-term efficacy and safety of locally-applied minocycline in adult periodontitis patients. J Clin Periodontol 1996; 23(8):707-16. doi: 10.1111/j.1600-051x.1996.tb00599.x [Crossref] [ Google Scholar]
- Murayama Y, Nomura Y, Yamaoka A, Ueda M, Hori T, Minabe M. [Local administration of minocycline for periodontitis Double blind comparative study of LS-007]. Nihon Shishubyo Gakkai Kaishi 1988; 30(1):206-22. doi: 10.2329/perio.30.206.[Japanese] [Crossref] [ Google Scholar]
- Pathak R, Bhatt S, Punetha VD, Punetha M. Chitosan nanoparticles and based composites as a biocompatible vehicle for drug delivery: a review. Int J Biol Macromol 2023; 253(Pt 7):127369. doi: 10.1016/j.ijbiomac.2023.127369 [Crossref] [ Google Scholar]
- Zwittnig K, Kirnbauer B, Jakse N, Schlenke P, Mischak I, Ghanaati S. Growth factor release within liquid and solid PRF. J Clin Med 2022; 11(17):5070. doi: 10.3390/jcm11175070 [Crossref] [ Google Scholar]
- Wong CC, Huang YM, Chen CH, Lin FH, Yeh YY, Bai MY. Cytokine and growth factor delivery from implanted platelet-rich fibrin enhances rabbit Achilles tendon healing. Int J Mol Sci 2020; 21(9):3221. doi: 10.3390/ijms21093221 [Crossref] [ Google Scholar]
- Lim CY, In J. Considerations for crossover design in clinical study. Korean J Anesthesiol 2021; 74(4):293-9. doi: 10.4097/kja.21165 [Crossref] [ Google Scholar]
- Pandit N, Dahiya R, Gupta R, Bali D, Kathuria A. Comparative evaluation of locally delivered minocycline and metronidazole in the treatment of periodontitis. Contemp Clin Dent 2013; 4(1):48-53. doi: 10.4103/0976-237x.111615 [Crossref] [ Google Scholar]
- Sachdeva S, Agarwal V. Evaluation of commercially available biodegradable tetracycline fiber therapy in chronic periodontitis. J Indian Soc Periodontol 2011; 15(2):130-4. doi: 10.4103/0972-124x.84381 [Crossref] [ Google Scholar]
- Nandan B, Barman Roy D, Pant VA, Gupta V, Bhaduria U, Kaur H. Comparative evaluation of cost-effectiveness, clinical and microbiological parameters of systemic antibiotics versus local drug delivery in aggressive periodontitis. Cureus 2022; 14(1):e20985. doi: 10.7759/cureus.20985 [Crossref] [ Google Scholar]
- Taalab MR, Mahmoud SA, El Moslemany RM, Abdelaziz DM. Intrapocket application of tea tree oil gel in the treatment of stage 2 periodontitis. BMC Oral Health 2021; 21(1):239. doi: 10.1186/s12903-021-01588-y [Crossref] [ Google Scholar]
- Narendran N, Anegundi RV, Shenoy SB, Chandran T. Autologous platelet-rich fibrin as an adjunct to non-surgical periodontal therapy-a follow up clinical pilot study. Wound Repair Regen 2022; 30(1):140-5. doi: 10.1111/wrr.12979 [Crossref] [ Google Scholar]
- Kadadasu R, Atchuta A, Palaparthy R, Reddy SH, Sisinty V, Beeravolu M. Clinicomicrobiological evaluation of the efficacy of local delivery of moxifloxacin and ibuprofen gel as an adjunct to scaling and root planing in chronic periodontitis patients. J Oral Maxillofac Pathol 2020; 24(1):26-32. doi: 10.4103/jomfp.JOMFP_253_19 [Crossref] [ Google Scholar]
- Shunmuga PD, Tadepalli A, Parthasarathy H, Ponnaiyan D, Cholan PK, Ramachandran L. Clinical evaluation of the combined efficacy of injectable platelet-rich fibrin along with scaling and root planing in the non-surgical periodontal therapy of stage III and grade C periodontitis patients having type 2 diabetes mellitus: a randomized controlled trial. Clin Adv Periodontics. 2023. 10.1002/cap.10266.
- Shujaa Addin A, Akizuki T, Matsuura T, Hoshi S, Ikawa T, Maruyama K. Histological healing after nonsurgical periodontal treatment with enamel matrix derivatives in canine experimental periodontitis. Odontology 2018; 106(3):289-96. doi: 10.1007/s10266-018-0347-4 [Crossref] [ Google Scholar]