J Adv Periodontol Implant Dent. 16(1):4-8.
doi: 10.34172/japid.2024.012
Research Article
Comparative assessment of the physical structure of antler and bovine bone substitutes: An in vitro study
Mohammad Hossein Mahboubian Investigation, Resources, Visualization, Writing – original draft, 1, 2 
Mahdi Kadkhodazadeh Conceptualization, Methodology, Resources, Software, Supervision, Validation, Writing – review & editing, 1, 2 
Reza Amid Methodology, Resources, Writing – review & editing, 1, 2 
Anahita Moscowchi Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing, 1, 2, * 
Author information:
1Dental Research Center, Research Institute for Dental Sciences, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Periodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Background.
The use of bone graft materials has significantly increased. Given the inherent variations in structure and functionality between different grafting materials, this evaluated and compared the physical attributes of antler and bovine femur bone substitutes.
Methods.
In the present in vitro investigation, the surface morphological architecture of the two bone substitutes with different origins was assessed through scanning electron microscopy. Furthermore, the Brunauer–Emmett–Teller (BET) technique was employed to measure the porosity, specific surface area (SSA), and pore morphology.
Results.
Scanning electron microscopy observations indicated that the surface of the bovine particles appeared smoother, while the antler particles exhibited a rougher surface texture. The BET analysis revealed that both samples exhibited identical pore morphology. The SSA was 15.974 m2/g in the antler particles compared with 18.404 m2/g in the bovine sample. The total porosity volume in the antler and bovine femur bone substitutes were 0.2172 cm3/g and 0.2918 cm3/g, respectively. Additionally, the antler particles had a porosity percentage of 40%, whereas the bovine femur bone substitute showed a porosity percentage of 43.5%.
Conclusion.
Based on the results of this study, it seems that the two samples of bone grafting materials have comparable physical structures.
Keywords: Biocompatible materials, Bone substitutes, Physical phenomena
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The reconstruction of bone defects resulting from trauma, inflammatory diseases, and tumors holds significant importance1 due to the potential consequences of leaving these lesions untreated, which may result in the formation of connective tissue.2 Consequently, addressing bone defects is crucial to any clinical procedure.3
Despite having defined the ideal characteristics for a bone graft material over three decades ago, no material has been able to encompass all of these characteristics to date.4 The use of autogenous bone is widely regarded as the gold standard.5 In addition to proper histocompatibility, autografts do not elicit the immune response and possess essential properties for successful regeneration, including osteogenesis, osteoinduction, and osteoconduction.6 However, the use of autogenous bone presents challenges such as prolonged surgical time, potential complications in the donor site, and postoperative pain and discomfort. In addition, the potential for infection transmission and immune system stimulation associated with allografts has prompted the exploration of alternative biomaterials, such as xenografts and synthetic materials, to treat bone defects.6-8
Numerous studies have demonstrated that using biological sources to prepare hydroxyapatite is a valuable approach for producing inexpensive and effective xenografts for bone regeneration.9 The key advantage of xenografts is their unrestricted availability. In addition, they possess biocompatibility, a porous structure, reasonable production costs, and mechanical strength.10
Xenografts can be obtained from different species,11 with commonly available commercial xenograft products typically sourced from bovine origins that may raise ethical concerns due to the sacrifice of involved animals.12 The antler, unique to mammals, is the sole body part capable of complete regeneration and exhibits a remarkable growth rate of 2 to 4 cm per day.13 The physical and structural characteristics of antlers have garnered significant interest.14
The biological response to transplant materials may be influenced by their physical attributes, including porosity, particle size, and shape.15 Therefore, this study aimed to investigate and compare the physical and structural characteristics of two bone substitutes derived from antler and bovine femur sources.
Methods
This in vitro study was carried out using particles with bovine femur bone origin (Bone+ B®, Novateb Pars Co., Iran) and a bone substitute derived from Cervus elaphus Maral’s antler (Maral Pajoohesh Shams Co., Iran).
The morphological characteristics were assessed using a scanning electron microscope (SEM) with an operating voltage of 25.0 kV (TESCAN VEGA 3, TSCAN, Brno, Czech Republic). Porosity and specific surface area (SSA) were determined through Brunauer-Emmett-Teller (BET) analysis using 0.5 grams of each bone material. The bone substitutes were pretreated and degassed by vacuuming for 2 hours (FlowPrep 060, Micromeritics, GA, USA), with liquid nitrogen as the adsorptive. The saturated vapor pressure was set at 88 kilo Pascal, and the temperature was set at 77 K (TriStar II Plus, Micromeritics, GA, USA). SSA was expressed as square meters per gram of mineral (m2/g). The shape of the pores was determined through an adsorption-desorption diagram, while the porosity volume and mean pore diameter were measured using the BET equation. Both assays were conducted and reported by an individual blinded to the characteristics of the materials.
Results
The morphological analysis revealed that the antler-derived bone substitute exhibited more rounded angles than the bovine particles. The bovine grafting material particles displayed sharper and more fragmented edges (Figure 1). The particle size of the antler particles encompassed a wider range than the material derived from bovine femur bone (Figure 2). Furthermore, greater variation in the shape and size of particles was observed in the antler particles. The surface of the particles derived from bovine femur bone was smoother, while the antler particles exhibited more surface roughness. Both materials displayed holes ranging approximately 50‒300 µm in size. Layered structures were observed in both samples, with the grafting material derived from bovine femur bone exhibiting more pronounced visibility. Small nodules were present in both samples, but the antler sample exhibited a significantly higher density and larger size of these nodules. The majority of particles in both samples fell within a range of 300‒600 µm, with no particles smaller than 150 µm in either sample. Only the sample derived from antler origin contained particles with dimensions ranging from 1000 to 2000 µm.
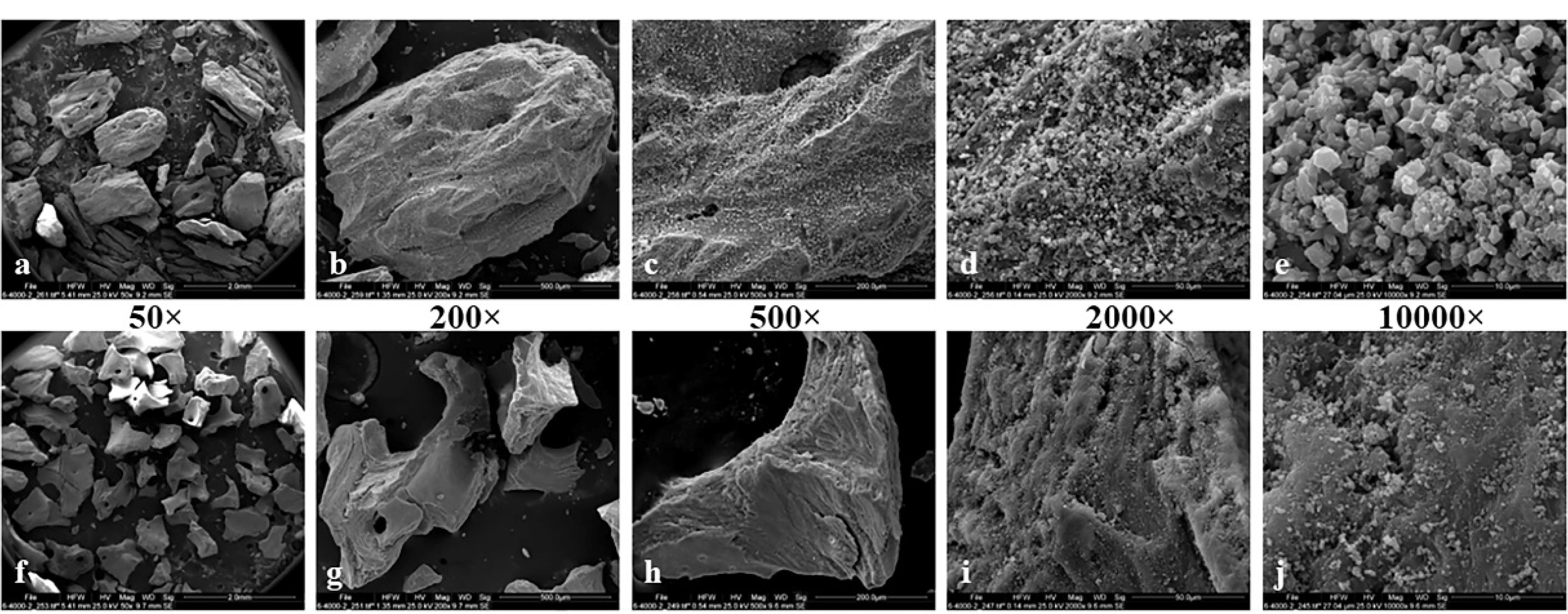
Figure 1.
Surface morphology of bone substitutes: Antler (a-e) and Bovine (f-j). Magnification: a and f ( × 50), b and g ( × 200), c and h ( × 500), d and h ( × 2,000), e and j ( × 10000)
.
Surface morphology of bone substitutes: Antler (a-e) and Bovine (f-j). Magnification: a and f ( × 50), b and g ( × 200), c and h ( × 500), d and h ( × 2,000), e and j ( × 10000)
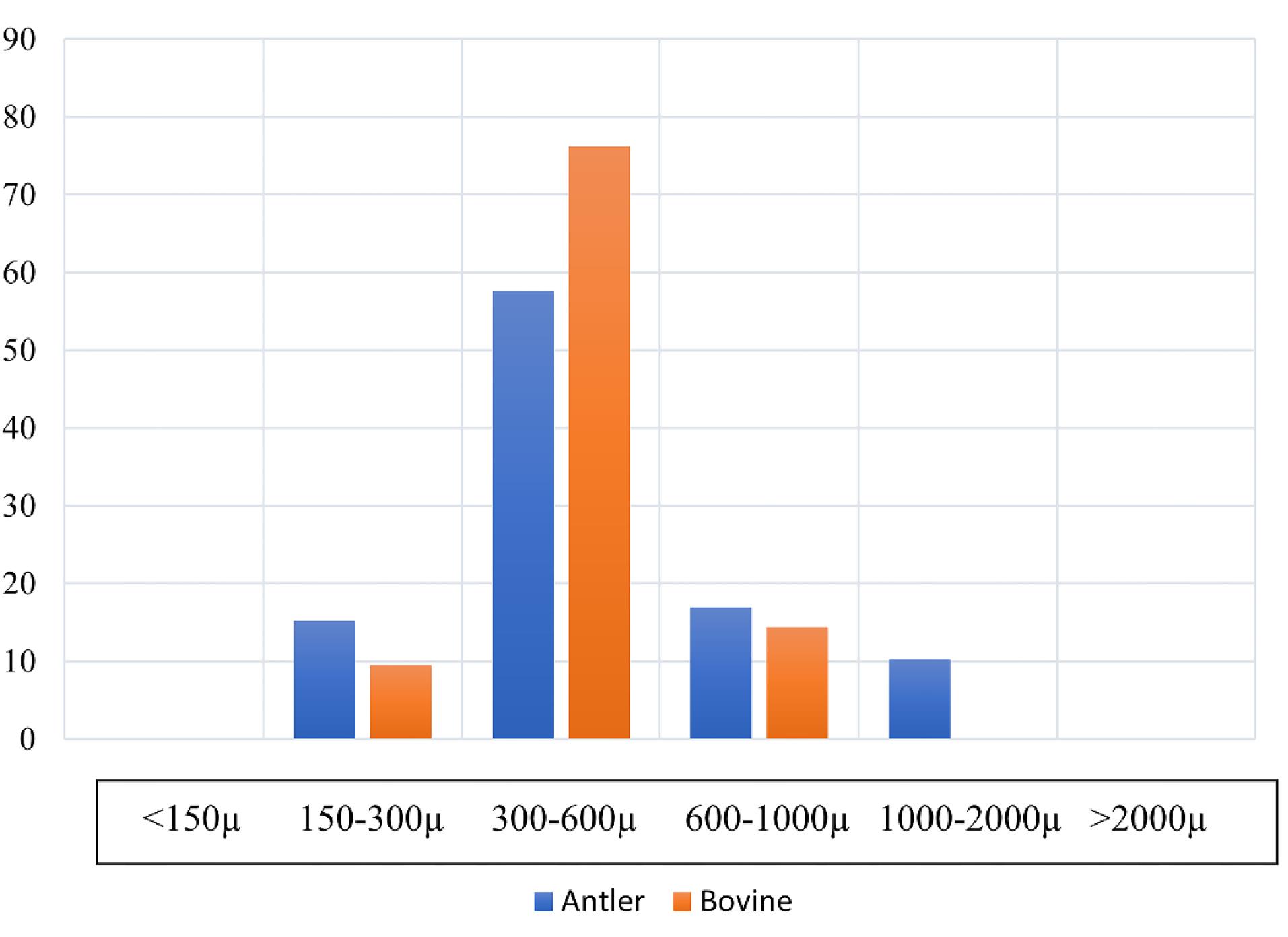
Figure 2.
Particle size distribution of the bone substitutes
.
Particle size distribution of the bone substitutes
The pore morphology in both bone substitutes exhibited a slit-like structure. Table 1 presents the results of the BET analysis. The distribution of pores by diameter is depicted in the Barrett-Joyner-Halenda (BJH) diagram (Figure 3).
Table 1.
Porosity and specific surface area
|
Type of bone substitute
|
Porosity (%)
|
Density (g/cm3)
|
Specific surface area (m2/g)
|
Total porosity volume (cm3/g)
|
Mean hole diameter (nm)
|
| Antler |
040 |
0.55 |
15.974 |
0.2172 |
54.377 |
| Bovine |
43.5 |
0.67 |
18.404 |
0.2918 |
63.41 |
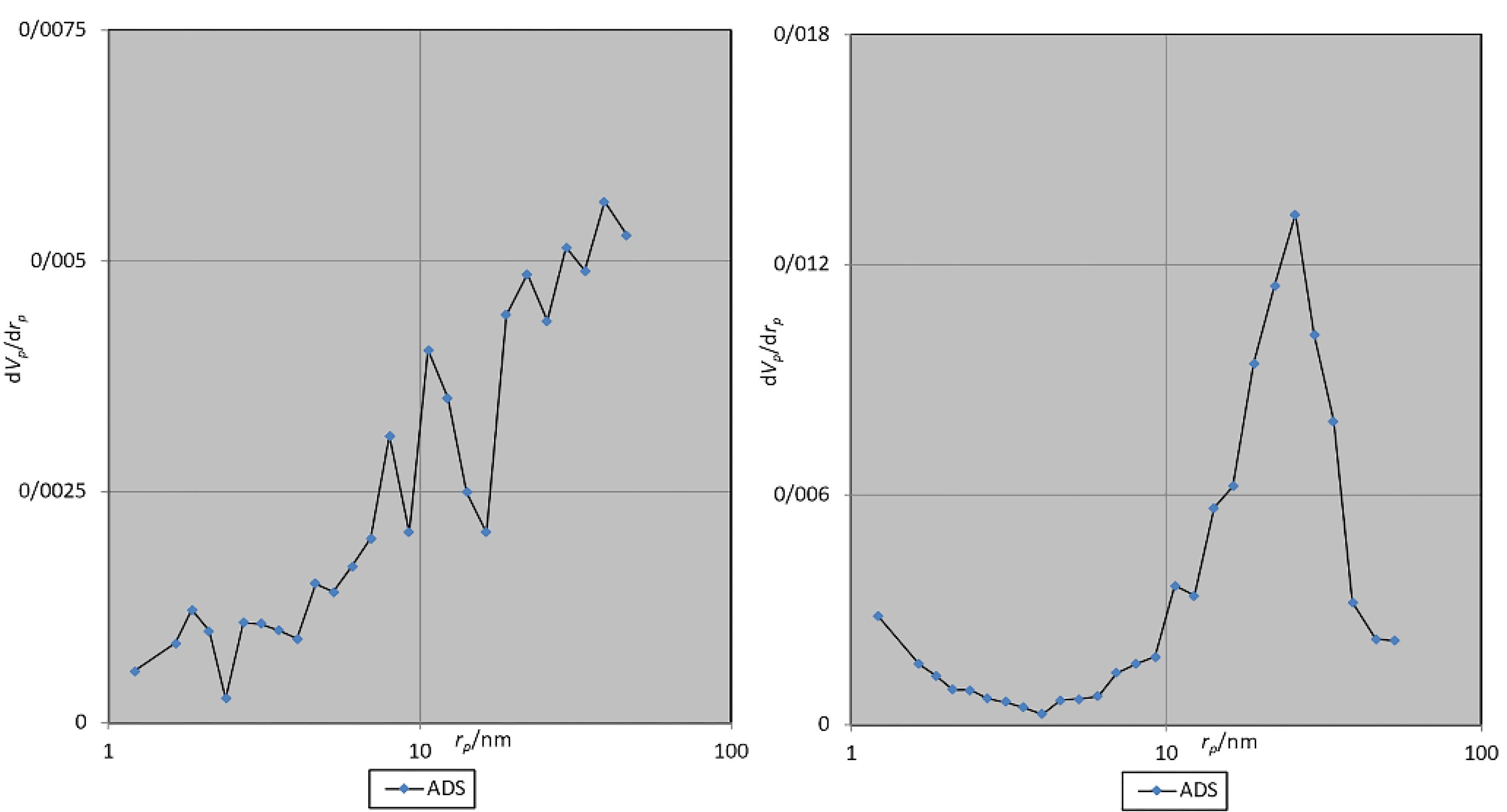
Figure 3.
BJH diagram. Antler (left) and bovine (right)
.
BJH diagram. Antler (left) and bovine (right)
Discussion
SEM revealed that the antler particles had more diverse sizes, which might be attributed to differences in their respective preparation methods. The majority of large particles in both samples exhibited holes ranging in size from 50 to 300 µm, which is consistent with the particle size of other xenografts. It has been demonstrated that cavities larger than 100 µm are crucial for the formation of blood vessels.16 A wide range of particle sizes might impede the angiogenesis process, as smaller particles tend to fill the interstitial spaces between larger particles and slow down this process.17 Furthermore, the antler particles had rougher surfaces. Previous research has demonstrated that smaller particles and a rougher surface of grafting materials elicit a stronger immune response, characterized by increased production of TNF-a and IL-6.18 This immune response is associated with the recruitment of cells to the regeneration site and the replacement of the graft material with new bone. Additionally, a rougher surface promotes better adhesion of osteoblasts, a stronger connection between the host bone and the graft material, and improved bone regeneration.19 The expression of osteoprotegerin, a receptor related to osteoclastogenesis, is likely to be higher in the antler particles due to surface roughness.20,21 The bovine particles had sharper angles, which might potentially result in Schneiderian membrane perforation during sinus augmentation procedures.
Porosity in grafting substitutes allows the infiltration of cells into the material. The presence of these pores helps nourish and dispose of osteoblasts’ waste materials. The minimum size of porosity in bone graft materials is typically around 100 µm. However, the optimal size for porosity among materials is > 300 µm to ensure proper blood supply.22-24 Another crucial factor to consider is the surface characteristics of the graft material, as they significantly influence angiogenesis, the interconnection of bone cells, and their migration and proliferation.4 Furthermore, the impact of the origin of xenograft on its properties has been demonstrated.25
Different techniques are employed to assess the porosity and specific surface of materials. The measurement of porosity with a size range of 1‒100 µm is accomplished using the mercury-assisted porosity measurement. On the other hand, the BET procedure is a precise method for evaluating pores within a range of 1‒100 nm. Alternatively, low-angle x-ray and neutron scattering approaches can be used to evaluate pores ranging from 0.4 to 2 nm.26 Given the necessity to examine the porosity of the samples at dimensions below 100 nm, the gas absorption method and BET theory were employed in the present study.
The antler sample exhibited a lower percentage of porosity (40%) compared to the bovine bone substitute (43.5%). Notably, both materials demonstrated a lower porosity percentage than Bio-Oss (70.5%).25 In Zhang’s investigation, the porosity of the prepared xenograft from deer antler was 75%, which surpasses the porosity of the test material in this study.27 It is important to acknowledge that the animal’s preparation procedure, breed, and age may influence the porosity level. Furthermore, it is imperative to consider that the testing protocol might impact the outcomes.
The BET analysis revealed that the bovine bone substitute micropores were, on average, 16.6% larger than those of the antler particles. These micropores in both materials are slightly larger and comparable to that of Bio-Oss (30 nm).28 Examining the pore size distribution, the antler sample exhibited greater dispersion, with a higher percentage of pores under 10 nm. The SSA in both samples was much higher than Puros (2 m2/g) and Creos allografts (0.025 m2/g).29 The sample obtained from bovine bone demonstrated a 34% increase in pore volume and a SSA 15.2% greater per gram compared to the antler sample. Consequently, due to its greater SSA, higher porosity percentage, and wider pore size, it appears that the bovine sample will undergo resorption at a faster rate. Bone graft materials that exhibit slower degradation are better suited for alveolar crest reconstruction, albeit necessitating a longer duration for repair.30
The pores in both samples exhibited a continuous transverse extension within a single particle, displaying a slit-like configuration. This particular pore structure has been demonstrated to facilitate enhanced molecular and fluid mobility. Consequently, it appears that the morphology of the pores in both materials may contribute to intercellular signaling.31
Further research should be conducted to compare this particular bone substitute with other grafting materials currently available on the market or other bone materials with different processing procedures. In addition, it is recommended to assess and compare the impact of the physical attributes of these two bone substitutes in animal studies, specifically in terms of osteopromotion levels, material absorption rates, replacement with new bone, and angiogenesis.
Conclusion
Based on the results of this study, it seems that the two samples of bone substitute originating from antler and bovine femur bone have similar physical structures. Both materials’ porosity, SSA, and density were almost identical. The bovine material displayed a limited range of particle sizes. On the other hand, the antler bone substitute particles exhibited a rougher surface texture that might enhance osteoblast adhesion.
Consent for Publication
Not applicable.
Competing Interests
The authors do not have any financial interest in the companies whose materials were included in this study.
Data Availability Statement
The data will be shared upon reasonable request by the corresponding author.
Ethical Approval
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.DRC.REC.1401.002).
Funding
The study was funded by Shahid Beheshti University of Medical Sciences.
References
- Kiany Yazdi F, Mostaghni E, Ansari Moghadam S, Faghihi S, Monabati A, Amid R. A comparison of the healing capabilities of various grafting materials in critical-size defects in guinea pig calvaria. Int J Oral Maxillofac Implants 2013; 28(5):1370-6. doi: 10.11607/jomi.2906 [Crossref] [ Google Scholar]
- Joschek S, Nies B, Krotz R, Göferich A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000; 21(16):1645-58. doi: 10.1016/s0142-9612(00)00036-3 [Crossref] [ Google Scholar]
- Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004; 25(6):987-94. doi: 10.1016/s0142-9612(03)00621-5 [Crossref] [ Google Scholar]
- Haugen HJ, Lyngstadaas SP, Rossi F, Perale G. Bone grafts: which is the ideal biomaterial?. J Clin Periodontol 2019; 46 Suppl 21:92-102. doi: 10.1111/jcpe.13058 [Crossref] [ Google Scholar]
- Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent 2017; 3(1):23. doi: 10.1186/s40729-017-0084-4 [Crossref] [ Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40(5):363-408. doi: 10.1615/critrevbiomedeng.v40.i5.10 [Crossref] [ Google Scholar]
- Calasans-Maia MD, de Almeida Barros Mourão CF, Alves AT, Sartoretto SC, de Uzeda MJ, Granjeiro JM. Maxillary sinus augmentation with a new xenograft: a randomized controlled clinical trial. Clin Implant Dent Relat Res 2015; 17 Suppl 2:e586-93. doi: 10.1111/cid.12289 [Crossref] [ Google Scholar]
- Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg 2012; 40(8):706-18. doi: 10.1016/j.jcms.2012.01.002 [Crossref] [ Google Scholar]
- Akram M, Ahmed R, Shakir I, Wan Ibrahim WA, Hussain R. Extracting hydroxyapatite and its precursors from natural resources. J Mater Sci 2014; 49(4):1461-75. doi: 10.1007/s10853-013-7864-x [Crossref] [ Google Scholar]
- Diomede F, D’Aurora M, Gugliandolo A, Merciaro I, Orsini T, Gatta V. Biofunctionalized scaffold in bone tissue repair. Int J Mol Sci 2018; 19(4):1022. doi: 10.3390/ijms19041022 [Crossref] [ Google Scholar]
- Karampas IA, Orkoula MG, Kontoyannis CG. Effect of hydrazine based deproteination protocol on bone mineral crystal structure. J Mater Sci Mater Med 2012; 23(5):1139-48. doi: 10.1007/s10856-012-4593-7 [Crossref] [ Google Scholar]
- Amirfeyz R, Stanley D. Allograft-prosthesis composite reconstruction for the management of failed elbow replacement with massive structural bone loss: a medium-term follow-up. J Bone Joint Surg Br 2011; 93(10):1382-8. doi: 10.1302/0301-620x.93b10.26729 [Crossref] [ Google Scholar]
- Chen PY, Stokes AG, McKittrick J. Comparison of the structure and mechanical properties of bovine femur bone and antler of the North American elk (Cervus elaphus canadensis). Acta Biomater 2009; 5(2):693-706. doi: 10.1016/j.actbio.2008.09.011 [Crossref] [ Google Scholar]
- Launey ME, Chen PY, McKittrick J, Ritchie RO. Mechanistic aspects of the fracture toughness of elk antler bone. Acta Biomater 2010; 6(4):1505-14. doi: 10.1016/j.actbio.2009.11.026 [Crossref] [ Google Scholar]
- Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001; 22(13):1705-11. doi: 10.1016/s0142-9612(00)00305-7 [Crossref] [ Google Scholar]
- Kuboki Y, Jin Q, Kikuchi M, Mamood J, Takita H. Geometry of artificial ECM: sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect Tissue Res 2002; 43(2-3):529-34. doi: 10.1080/03008200290001104 [Crossref] [ Google Scholar]
- Anil A, Sadasivan A, Koshi E. Physicochemical characterization of five different bone graft substitutes used in periodontal regeneration: an in vitro study. J Int Soc Prev Community Dent 2020; 10(5):634-42. doi: 10.4103/jispcd.JISPCD_263_20 [Crossref] [ Google Scholar]
- Al Ruhaimi KA. Bone graft substitutes: a comparative qualitative histologic review of current osteoconductive grafting materials. Int J Oral Maxillofac Implants 2001; 16(1):105-14. [ Google Scholar]
- Leteve M, Passuti N. Current concepts in bone graft substitutes. New Journal of Glass and Ceramics 2018; 8(3):39-54. doi: 10.4236/njgc.2018.83004 [Crossref] [ Google Scholar]
- Jung S, Bohner L, Hanisch M, Kleinheinz J, Sielker S. Influence of implant material and surface on differentiation and proliferation of human adipose-derived stromal cells. Int J Mol Sci 2018; 19(12):4033. doi: 10.3390/ijms19124033 [Crossref] [ Google Scholar]
- Liu W, Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol Med Rep 2015; 11(5):3212-8. doi: 10.3892/mmr.2015.3152 [Crossref] [ Google Scholar]
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005; 26(27):5474-91. doi: 10.1016/j.biomaterials.2005.02.002 [Crossref] [ Google Scholar]
- Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010; 31(3):461-6. doi: 10.1016/j.biomaterials.2009.09.063 [Crossref] [ Google Scholar]
- Saito E, Saito A, Kuboki Y, Kimura M, Honma Y, Takahashi T. Periodontal repair following implantation of beta-tricalcium phosphate with different pore structures in class III furcation defects in dogs. Dent Mater J 2012; 31(4):681-8. doi: 10.4012/dmj.2011-259 [Crossref] [ Google Scholar]
- Lee JH, Yi GS, Lee JW, Kim DJ. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J Periodontal Implant Sci 2017; 47(6):388-401. doi: 10.5051/jpis.2017.47.6.388 [Crossref] [ Google Scholar]
- Fraissard JP. Physical Adsorption: Experiment, Theory, and Applications. 1st ed. Dordrecht: Springer; 2012.
- Zhang X, Cai Q, Liu H, Heng BC, Peng H, Song Y. Osteoconductive effectiveness of bone graft derived from antler cancellous bone: an experimental study in the rabbit mandible defect model. Int J Oral Maxillofac Surg 2012; 41(11):1330-7. doi: 10.1016/j.ijom.2012.05.014 [Crossref] [ Google Scholar]
- Figueiredo M, Henriques J, Martins G, Guerra F, Judas F, Figueiredo H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J Biomed Mater Res B Appl Biomater 2010; 92(2):409-19. doi: 10.1002/jbm.b.31529 [Crossref] [ Google Scholar]
- Ajami E, Fu C, Park SJ, Wang X, Wen HB. Comparison of the effects of tissue processing on the physicochemical properties of bone allografts. Int J Oral Maxillofac Implants 2023; 38(1):169-80. doi: 10.11607/jomi.9781 [Crossref] [ Google Scholar]
- Wach T, Kozakiewicz M. Fast-versus slow-resorbable calcium phosphate bone substitute materials-texture analysis after 12 months of observation. Materials (Basel) 2020; 13(17):3854. doi: 10.3390/ma13173854 [Crossref] [ Google Scholar]
- Ceratti DR, Faustini M, Sinturel C, Vayer M, Dahirel V, Jardat M. Critical effect of pore characteristics on capillary infiltration in mesoporous films. Nanoscale 2015; 7(12):5371-82. doi: 10.1039/c4nr03021d [Crossref] [ Google Scholar]