J Adv Periodontol Implant Dent. 17(1):54-58.
doi: 10.34172/japid.025.3502
Case Report
Treatment of an endodontic-periodontal lesion using peripheral blood mesenchymal stem cells (PBMSCs) and platelet-rich fibrin matrix (PRFM): A case report
Sphoorthi Anup Belludi Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing, 1, * 
Sharaz Shaik Conceptualization, Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing, 2
Neha Pradhan Investigation, Validation, Visualization, Writing – review & editing, 1
Sreeparvathy Rema Project administration, Supervision, Validation, Visualization, Writing – review & editing, 1
Author information:
1Department of Periodontics, K.L.E Society’s Institute of Dental Sciences, Bengaluru, Karnataka, India
2Lincoln University PhD Program, Lenora Institute of Dental Science, Rajahmundry, Andhra Pradesh, India
Abstract
In the current report, we discuss the available treatment options and present a successfully treated periodontal-endodontic lesion using autogenous peripheral blood mesenchymal stem cells (PBMSCs) and platelet-rich fibrin matrix (PRFM). A patient presented with a complaint of food impaction and bad breath. Clinically, the lower right first molar was non-vital and had a deep periodontal pocket and attachment loss. Radiographically, the distal root had an angular bone loss extending to the apex. The endodontic condition was treated with chemomechanical debridement, calcium hydroxide dressing, and obturation. Later, we reflected a full-thickness mucoperiosteal flap and thoroughly debrided the granulation tissue. We filled the defect with a gel containing PBMSCs and PRFM, prepared from the patient’s peripheral blood, and sutured the flap. After nine months, we noticed significant osseous fill and 5 mm of gain in the clinical attachment level. The outcomes of the case show the periodontal regenerative potential of the novel combination.
Keywords: Endo-periodontal lesion, Peripheral blood stem cells, Platelet-rich fibrin, Regenerative endodontics
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
The concomitant presence of inflammatory periodontal disease and pulpal pathosis complicates the diagnosis and treatment planning of endodontic-periodontal lesions (EPLs). Hence, EPLs usually need a multidisciplinary approach.1,2 The aim of treating the periodontal pocket is to regain the periodontal attachment; it may range from oral prophylaxis to regenerative procedures. Currently, much interest has been observed in autologous platelet/fibrin biologics. According to Dohan’s classification, our material of interest is pure platelet-rich fibrin/platelet-rich fibrin matrix (PRFM).3,4
In alignment with regenerative dentistry and the current concept of tissue engineering, stem cells can be an effective module due to their pluripotency and regenerative ability.5 The extraction of MSCs from bone marrow and other sources is an invasive, high-risk approach that provides a low-frequency and heterogeneous population. Peripheral blood is possibly the most straightforward source due to the accessibility and lack of donor-organ morbidity in comparison to other sources (bone marrow, umbilical cord, adipose tissue, salivary glands, periosteum, periodontium, and dental pulp) of mesenchymal stem cells (MSCs). Despite numerous studies on using other sources of stem cells in periodontal regeneration,5 there is a paucity of reports on the application of peripheral blood-derived mesenchymal stem cells (PBMSCs). The current case report describes a novel approach using PBMSCs + PRFM en masse to treat an EPL patient.
Case Presentation
A healthy patient (male, age = 42 years) presented with a chief complaint of discomfort and food impaction in the lower right back teeth region and bad breath for five months. Intraoral examination revealed a full constituent dentition, generalized moderate accumulation of dental biofilm, calculus, and generalized bleeding on probing, more prominent in the lower right second premolar to the third molar region (#45 to #48). Food impaction was observed between the lower right first and second molars (#46 and #47). Mild tenderness on percussion (TOP) was elicited with #46 and #47. Grade I mobility and occlusal and buccal caries were detected with #46. The clinical attachment level (CAL) and probing pocket depth (PPD) were 8 mm in the interdental region of #47 and #48 and 4 mm between #45 and #46. However, the CAL was 9 mm, and PPD was 8 mm in the interdental region of #46 and #47 due to 1 mm of gingival recession on the distobuccal root of #46 (Table 1). A long-cone paralleling technique and intraoral direct digital periapical radiovisiograph (RVG-Suni Medical Imaging, Apteryx Inc., Acron, Ohio, USA) were used for radiographic evaluation (Table 2). The radiographs revealed angular bone loss in the lower right first and second molar (#46 and #47) region, with the base of the vertical defect extending to the apex of the distal root of the lower right first molar (#46); however, there was no sign of root damage (Figure 1). Vitality test exhibited a delayed response with #46 and a positive response with #47. The patient was devoid of complicating factors such as diabetes and smoking, which could have affected the healing and treatment outcome. Based on the presentations and common classification by Simon et al.,6 the lesion was classified as primary periodontal lesions with secondary endodontic involvement and grade 3 endo-periodontal lesion in periodontitis patients as per the 2017 World Workshop Classification.7
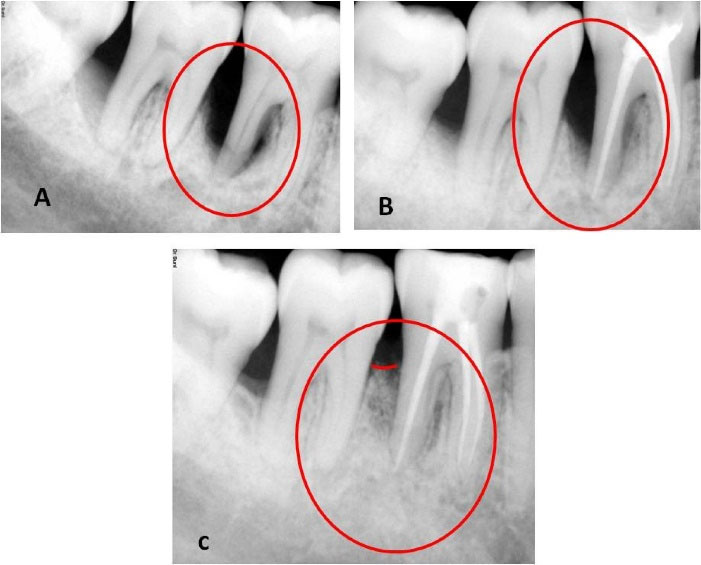
Figure 1.
Preoperative, postoperative, and follow-up radiographs. (A) Preoperative radiograph displaying endodontic-periodontal lesion. (B) Three-month follow-up displaying partial bone regain. (C) Nine-month follow-up displaying a significant bone regain
.
Preoperative, postoperative, and follow-up radiographs. (A) Preoperative radiograph displaying endodontic-periodontal lesion. (B) Three-month follow-up displaying partial bone regain. (C) Nine-month follow-up displaying a significant bone regain
Treatment
Regarding the clinical and radiographic findings, the provisional prognosis was considered fair. The treatment protocol was planned in 4 phases8: (1) preliminary management, (2) endodontic management, 3: PBMSCs + PRFM gel preparation and surgical procedure, and (4) follow-up management.
Preliminary management and patient consent
We performed oral prophylaxis, educated the patient about the importance of oral hygiene, and demonstrated the use of dental floss and chlorhexidine mouthwash. Since the patient did not have acute symptoms, we did not prescribe oral medications. We explained the current condition of the lower right first molar (#46) and our proposal to use the novel treatment procedure. The patient voluntarily gave consent for the procedure. Written informed consent was obtained from the patient for enrollment into the treatment protocol and for publishing the acquired data. Ethical clearance for the treatment was received from the Institutional Ethics Committee (Blinded for review).
Endodontic management
Root canal treatment incorporated chemomechanical debridement under local anesthesia and isolation using rotary endodontic instruments (ProTaper Next, Dentsply Sirona) and filling of the root canals with calcium hydroxide paste (ApexCal® Ivoclar Vivadent) using a lentulo spiral. The root canals were obturated by lateral condensation of gutta-percha (ProTaper Next Gutta Percha endodontic points, Dentsply, Maillefer) and a calcium hydroxide-based sealer (Apexit Plus Root Canal Sealer, Ivoclar Vivadent Inc.). A permanent composite resin restoration was placed to seal off the access cavity.
PBMSCs + PRFM gel preparation9
PBMSCs and PRFM were obtained from the peripheral blood by Merisis Supercell concentrate, DiponEd BioIntelligence© and Merisis PRFM kit, Merisis Biological Devices, and DiponEd BioIntelligence©, respectively, by following manufacturer kit insert instructions (Figure 2).9
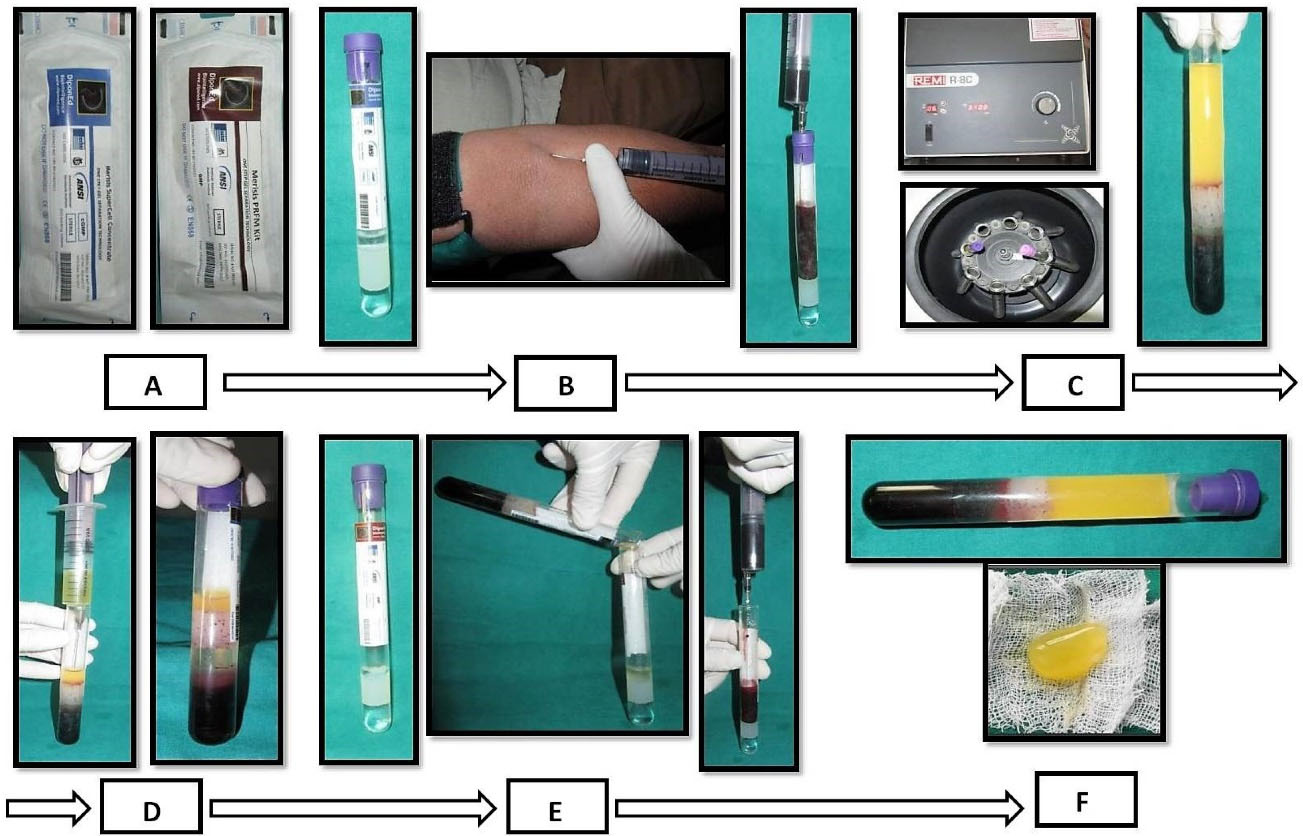
Figure 2.
Procurement and preparation of PBMSCs + PRFM en masse. (A) Merisis kit for PRFM and Merisis Supercell concentrate kit for PBMSCs. (B) Aspiration of blood for stem cell preparation. (C) Centrifugation of blood in a laboratory centrifuge. (D) Extraction of PBMSCs from the uppermost layer of 0.5-1 mL of fluid from the kit. (E) The second aspiration of blood for PRFM preparation. (F) PRFM and PBMSCs en masse obtained
.
Procurement and preparation of PBMSCs + PRFM en masse. (A) Merisis kit for PRFM and Merisis Supercell concentrate kit for PBMSCs. (B) Aspiration of blood for stem cell preparation. (C) Centrifugation of blood in a laboratory centrifuge. (D) Extraction of PBMSCs from the uppermost layer of 0.5-1 mL of fluid from the kit. (E) The second aspiration of blood for PRFM preparation. (F) PRFM and PBMSCs en masse obtained
Surgical procedure
After anesthetizing with 2% lignocaine containing 1:80,000 adrenaline (Lignox A 2%, Indoco Remedies LTD., India) using the inferior alveolar nerve block. We placed sulcular incisions on both buccal and lingual sides and reflected a full-thickness mucoperiosteal flap extending from the mesial of the lower right second premolar (#45) to the mesial line angle of the lower right third molar (#48). Thorough debridement of granulation tissue in the intrabony defect (3-wall defect between #46 and #47 and #47 and #48 regions), scaling, and root planing of remnant calculus on the root surfaces was performed (Figure 3). After pre-suturing of the interdental papillae in the #46, #47, and later in #47 and #48 regions, the freshly prepared PBMSCs + PRFM was extracted en masse from the glass vial with the help of tweezers and filled into the intrabony defect as a sole material. The flaps were approximated by interrupted sutures using 4-0 silk material (Figure 3). No periodontal dressing was applied. Antibiotics (Amoxycillin 500 mg every 8 hours for 7 days) and analgesics (Ibuprofen 400 mg every 4 hours) as required for pain management were prescribed. 0.2% chlorhexidine rinses every 12 hours for 14 days were advised. Postoperative instructions were given, an ultra-soft toothbrush was prescribed, and the patient was advised to refrain from disturbing the surgical site for the next two days.
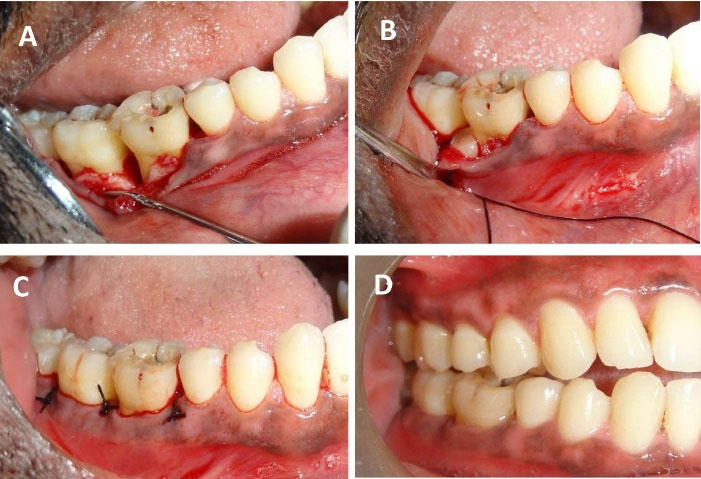
Figure 3.
Surgical procedure and post-surgical illustrations. (A) A mucoperiosteal flap was reflected, and surgical debridement was performed. (B) PBMSCs + PRFM en masse was placed in the intrabony defect. (C) Interrupted sutures were placed to approximate the flap. (D) Three-month postoperative follow-up displaying complete soft tissue healing
.
Surgical procedure and post-surgical illustrations. (A) A mucoperiosteal flap was reflected, and surgical debridement was performed. (B) PBMSCs + PRFM en masse was placed in the intrabony defect. (C) Interrupted sutures were placed to approximate the flap. (D) Three-month postoperative follow-up displaying complete soft tissue healing
Outcome and follow-up
The patient was followed for one month after endodontic treatment with no evident changes in the clinical parameters; hence, we proceeded to surgical therapy. We also conducted post-surgical follow-up after a week, one month, 3 months, and 9 months. During the first follow-up appointment, the patient had no complaints of pain and discomfort and no tenderness on percussion. We reinforced oral prophylactic instructions. The sutures were removed ten days after surgery. During the one-month and three-month appointments, the patient displayed good oral hygiene. The surgical area displayed complete healing, no tenderness on percussion, and no tooth mobility. Radiographic evaluation three months after surgery displayed a partial bone fill (Figure 1). During the 9-month follow-up appointment, the periodontal lesion had significantly improved clinically and radiographically (Figures 1 and 3). A significant reduction in the periodontal probing depth and gain in the clinical attachment level was noted (Table 1). At any of these intervals, there was no attempt at subgingival instrumentation.
Table 1.
Clinical attachment loss at right lower quadrant, measured in mm: a pre- and 9-month postoperative comparison
|
|
Tooth no.
|
|
#45
|
#46
|
#47
|
#48
|
|
Pre
|
Post
|
Pre
|
Post
|
Pre
|
Post
|
Pre
|
Post
|
| Buccal |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
| Lingual |
3 |
3 |
4 |
3 |
3 |
3 |
3 |
3 |
| Interdental |
M |
D |
M |
D |
M |
D |
M |
D |
M |
D |
M |
D |
M |
D |
M |
D |
| 3 |
4 |
2 |
3 |
4 |
9 |
3 |
4 |
8 |
8 |
4 |
4 |
7 |
3 |
3 |
3 |
Pre: preoperative parameters (in mm).
Post: 9-month postoperative parameters (in mm).
M: mesial, D: distal.
Discussion
A combined EPL does not respond with root canal treatment alone. The patient’s 4-week post-root canal treatment follow-up evaluation did not indicate any distinct improvement in the clinical parameters. Considering CAL deeper than the critical probing depth of 5.4 mm in our case, surgical intervention with regenerative therapy was deemed beneficial.10 In the current case, after initial biomechanical debridement, the canals were filled with calcium hydroxide endodontic dressing for a week, followed by obturation with gutta-percha and a calcium hydroxide-based sealer. A report suggests that pulpal infection tends to stimulate epithelial growth apically adjacent to the stripped dentinal surface, especially in the presence of periodontal infection.10,11 Open dentinal tubules, accessory canals, and apical foramen are the three possible transmission channels for contamination.12
In the present case, as the vitality test showed a delayed response, endodontic therapy was carried out to eliminate the partially necrotic pulp and prevent remnant nidus of infection and future necrosis and infection following periodontal regenerative surgery. Therefore, root canal treatment was completed before periodontal regenerative therapy. Furthermore, calcium hydroxide dressing in the root canal could promote periodontal healing in such cases.10 The overall success of an EPL usually depends on the efficiency of periodontal therapy.
Numerous biomaterials are currently available for periodontal regeneration. A study reported that a combination of PRP and stem cells resulted in better periodontal tissue regeneration than stem cell therapy alone13; furthermore, using PRFM instead of PRP could produce better results.14,15 PRFM enhances osteoblast differentiation and stimulates periodontal soft tissue regeneration.16 The influence of PRFM on periodontal regeneration can be accredited to transforming growth factor β, platelet-derived growth factor, insulin-like growth factor, and basic fibroblast growth factor.15,17 Apart from the healing potential of PRFM, it was shown that PRFM produced significantly higher mesenchymal stem cell proliferation.17 There are studies on the application of stem cells from various sources, more so on bone marrow mesenchymal stem cells;5 however, little is known on tissue regeneration by PBMSCs. PBMSCs have equivalent pluripotency and osteogenic ability to mesenchymal stem cells derived from bone marrow and umbilical cord.18,19 The stem cell-based periodontal regeneration could be mediated by monocyte chemoattractant protein-1, chemokine stromal cell-derived factor-1, chemokine stromal cell-derived factor-1, and exosomes cell-free strategy.20 Combining PRFM with PBMSCs would be more salutary than PRP as there is a more sustained release of growth factors by PRFM in comparison to PRP.21,22 Our approach of using PBMSCs + PRFM en masse in periodontal regeneration of EPL is novel as there are no similar reports in the current scientific literature. However, few studies suggest promising results for this combination as a regenerative material.9,13
The success rate of conventional modalities in the treatment of EPL combined lesions was reported to be very low (27‒37%) compared to a tooth survival rate of 92.31% at 5 years after a periodontal regenerative procedure.23,24Studies suggest that treatment outcomes seen at a first-year follow-up appointment tend to last for a long time.25 Our 9-month follow-up revealed an excellent treatment outcome. Uniformity in pre- and postoperative radiography and analysis were used to minimize the errors (Table 2). No bone graft or radiopaque material was used, which might mimic radiopaque bone fill in postoperative radiographs. Hence, the postoperative radiopacity can be considered a true osseous fill.
Table 2.
Standardized parameters used for pre- and postoperative radiographic evaluation
|
Parameter
|
Description
|
| Type of radiograph |
Intraoral direct digital periapical radiovisiograph |
| Radiographic equipment |
RVG-Suni Medical Imaging, Apteryx Inc., Acron, Ohio, USA. |
| Technique |
long cone paralleling technique |
| Exposure |
70 kVp, 7 ma for 0.2 seconds |
| The focus-to-film distance |
20 cm |
| Software for linear measurements |
Image J software, Wayne Rasband, National Institute of Health, USA |
Conclusion
A substantial osseous fill in the present study can be attributed to a definitive root canal treatment, periodontal debridement, and application of PRFM + PBMSCs. This protocol can be an easy, effective, and promising regenerative option. However, in vitro validity tests, histomorphometry studies, and large-scale randomized control trials are required to endorse the regenerative efficacy of this protocol.
Competing Interests
The authors do not have any financial interest in the companies whose materials are included in this article and have no conflicts of interest.
Consent for Publication
Not applicable.
Data Availability Statement
Not applicable.
Ethical Approval
We explained the current condition and our proposal for using the novel treatment procedure. The patient voluntarily gave consent for the procedure. Written informed consent was obtained from the patient for enrollment into the treatment protocol and for publishing the acquired data. Ethical clearance for the treatment was received from the Institutional Ethics Committee (IEC/Nov-2021/8).
References
- Tayal A, Ghosh S, Adhikari H D, Ghosh A. Management of an endo-perio lesion: a multidisciplinary approach. IP Indian J Conserv Endod 2021; 6(3):171-5. doi: 10.18231/j.ijce.2021.037 [Crossref] [ Google Scholar]
- Al Attas MA, Edrees HY, Sammani AM, Madarati AA. Multidisciplinary management of concomitant pulpal and periodontal lesion: a case report. J Taibah Univ Med Sci 2017; 12(5):455-60. doi: 10.1016/j.jtumed.2017.05.010 [Crossref] [ Google Scholar]
- Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014; 4(1):3-9. [ Google Scholar]
- Simon BI, Gupta P, Tajbakhsh S. Quantitative evaluation of extraction socket healing following the use of autologous platelet-rich fibrin matrix in humans. Int J Periodontics Restorative Dent 2011; 31(3):285-95. [ Google Scholar]
- Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, Freire M. Potential for stem cell-based periodontal therapy. J Cell Physiol 2016; 231(1):50-61. doi: 10.1002/jcp.25067 [Crossref] [ Google Scholar]
- Simon JH, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Periodontol 1972; 43(4):202-8. doi: 10.1902/jop.1972.43.4.202 [Crossref] [ Google Scholar]
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89 Suppl 1:S173-82. doi: 10.1002/jper.17-0721 [Crossref] [ Google Scholar]
- Oh SL, Fouad AF, Park SH. Treatment strategy for guided tissue regeneration in combined endodontic-periodontal lesions: case report and review. J Endod 2009; 35(10):1331-6. doi: 10.1016/j.joen.2009.06.004 [Crossref] [ Google Scholar]
- Singhal L, Belludi SA, Pradhan N, Manvi S. A comparative evaluation of the effect of platelet rich fibrin matrix with and without peripheral blood mesenchymal stem cells on dental implant stability: a randomized controlled clinical trial. J Tissue Eng Regen Med 2022; 16(4):422-30. doi: 10.1002/term.3290 [Crossref] [ Google Scholar]
- Blomlöf L, Lengheden A, Lindskog S. Endodontic infection and calcium hydroxide-treatment. Effects on periodontal healing in mature and immature replanted monkey teeth. J Clin Periodontol 1992; 19(9 Pt 1):652-8. doi: 10.1111/j.1600-051x.1992.tb01714.x [Crossref] [ Google Scholar]
- Jansson L, Ehnevid H, Blomlöf L, Weintraub A, Lindskog S. Endodontic pathogens in periodontal disease augmentation. J Clin Periodontol 1995; 22(8):598-602. doi: 10.1111/j.1600-051x.1995.tb00811.x [Crossref] [ Google Scholar]
- Rotstein I, Simon JH. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontol 2000 2004; 34:165-203. doi: 10.1046/j.0906-6713.2003.003431.x [Crossref] [ Google Scholar]
- Xu Q, Li B, Yuan L, Dong Z, Zhang H, Wang H. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J Tissue Eng Regen Med 2017; 11(3):627-36. doi: 10.1002/term.1953 [Crossref] [ Google Scholar]
- Lucarelli E, Beretta R, Dozza B, Tazzari PL, O’Connel SM, Ricci F. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater 2010; 20:13-23. doi: 10.22203/ecm.v020a02 [Crossref] [ Google Scholar]
- Walia KD, Belludi SA, Pradhan N, Jain V, Shaik S. Evaluation of platelet-rich fibrin matrix as a regenerative material in the surgical management of human periodontal intraosseous defects - a randomized controlled trial. Contemp Clin Dent 2022; 13(1):9-17. doi: 10.4103/ccd.ccd_832_20 [Crossref] [ Google Scholar]
- Li Q, Pan S, Dangaria SJ, Gopinathan G, Kolokythas A, Chu S. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res Int 2013; 2013:638043. doi: 10.1155/2013/638043 [Crossref] [ Google Scholar]
- Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol 2003; 74(6):849-57. doi: 10.1902/jop.2003.74.6.849 [Crossref] [ Google Scholar]
- Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 2012; 30(4):634-42. doi: 10.1002/jor.21556 [Crossref] [ Google Scholar]
- Trivanović D, Jauković A, Popović B, Krstić J, Mojsilović S, Okić-Djordjević I. Mesenchymal stem cells of different origin: comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci 2015; 141:61-73. doi: 10.1016/j.lfs.2015.09.019 [Crossref] [ Google Scholar]
- Suzuki K, Chosa N, Sawada S, Takizawa N, Yaegashi T, Ishisaki A. Enhancement of anti-inflammatory and osteogenic abilities of mesenchymal stem cells via cell-to-cell adhesion to periodontal ligament-derived fibroblasts. Stem Cells Int 2017; 2017:3296498. doi: 10.1155/2017/3296498 [Crossref] [ Google Scholar]
- Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen 2011; 19(6):753-66. doi: 10.1111/j.1524-475X.2011.00740.x [Crossref] [ Google Scholar]
- Belludi SA, Singhal L, Gubbala M. Peripheral blood mesenchymal stem cells and platelet rich fibrin matrix in the management of class II gingival recession: a case report. J Dent (Shiraz) 2021; 22(1):67-70. doi: 10.30476/dentjods.2020.81784.0 [Crossref] [ Google Scholar]
- Oh S, Chung SH, Han JY. Periodontal regenerative therapy in endo-periodontal lesions: a retrospective study over 5 years. J Periodontal Implant Sci 2019; 49(2):90-104. doi: 10.5051/jpis.2019.49.2.90 [Crossref] [ Google Scholar]
- Wong I, Ton A, Cassidy AJ, Fozzard N, Sharma LA, Love RM. A retrospective study on the prognostic factors and success, survival, and failure outcomes of treated endodontic-periodontal lesions. Clin Exp Dent Res 2024; 10(1):1-15. doi: 10.1002/cre2.848 [Crossref] [ Google Scholar]
- Marín-Botero ML, Domínguez-Mejía JS, Arismendi-Echavarría JA, Mesa-Jaramillo AL, Flórez-Moreno GA, Tobón-Arroyave SI. Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: a double-blind, randomized-clinical trial. Int Endod J 2006; 39(5):368-77. doi: 10.1111/j.1365-2591.2006.01081.x [Crossref] [ Google Scholar]