J Adv Periodontol Implant Dent. 2025;17(3):140-144.
doi: 10.34172/japid.025.3478
Research Article
Histologic evaluation of topical simvastatin effects on extraction sockets: A randomized controlled clinical trial
Nasrin Faal Rastegar Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing, 1 
Farzane Vaziri Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Seyed Mostafa Mahmoudi Conceptualization, Formal analysis, Methodology, Visualization, Writing – review & editing, 2 
Author information:
1Department of Periodontics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2Department of Oral Maxillofacial Pathology, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Abstract
Background.
The reduction of alveolar ridge volume after tooth extraction can be decreased through ridge preservation. According to previous studies, statin drugs induce osteogenesis. Therefore, this study aimed to evaluate the effect of simvastatin on the preservation and ossification of the alveolar ridge after tooth extraction.
Methods.
In this single-center randomized clinical trial, 40 dental sockets in 40 patients were randomly divided into the treatment group (collagen with simvastatin) and the control group (collagen only). Histologic bone examination was performed under a light microscope two months after socket preservation at the time of dental implants. The predictable variable was using simvastatin in dental sockets. In the treatment group, collagen was used with simvastatin; in the control group, only collagen was used. The percentage of bone formation was the primary outcome, which was measured as the area of newly formed bone. In this study, inflammatory reaction, the amount of remaining bone substitute, and foreign body reaction were compared between the two groups. Covariates included age, sex, and tooth location. T-test was used for normally distributed data, while the Mann–Whitney test was used for non-normal data. P<0.05 was considered significant.
Results.
The results showed that following eight weeks of simvastatin use in the treatment group, the percentage of new bone formation was significantly higher compared to the control group (treatment group vs. control group: 69.28±3.93 vs. 52.76±2.01; P=0.0001). No foreign body reaction and residual graft materials were observed in the treatment and control groups. Furthermore, the study showed an inflammatory reaction in only 23.5% of the samples in the control group (P=0.045).
Conclusion.
Simvastatin significantly increased the formation of new bone in the dental socket in the treatment group.
Keywords: Dental socket preservation, Histology, Simvastatin
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Tooth loss causes physiological and remodeling changes in the soft and hard tissues of the alveolar ridge, depending on multiple factors, including alveolar socket size, mucosal thickness, metabolic factors, and functional load.1,2 Bone resorption is inevitable, and implant placement is difficult unless steps are taken to preserve and regenerate it. Preservation of the alveolar ridge after surgery reduces residual ridge resorption and may improve implant placement from a functional and aesthetic viewpoint.3-6
Autogenous bone is the most predictable material for augmentation processes.7 However, bone donor resources are limited, and autogenous graft harvesting is associated with complications such as bleeding, pain, and infection.8 Bone graft substitutes reduce the complications of the donor site and increase the implant’s success rate.9,10
Statins, like simvastatin, are widely used drugs that reduce lipid levels. These drugs act through the mevalonate pathway. Several studies have shown that these drugs can regulate inflammatory responses through a mechanism independent of cholesterol reduction.11 Simvastatin is more desirable among statins since it can cross the cell membrane and has a shorter onset of action. It has the potential for osteoblast activation and osteoclast inhibition.They increase osteoblast differentiation by stimulating bone morphological proteins 2 (BMP-2).12-15 Administration of simvastatin is helpful in the healing of oral bone and cartilage.16
Previous studies have reported the use of simvastatin in a variety of lesions, such as the subgingival area in periodontal lesions,17 class II furcation involvement,18 subgingival areas in smokers with periodontitis,19 gingival areas in patients with type II diabetes,20 and human maxillary sinus.21
The potential of these drugs in soft tissue healing and TMJ arthritis has also been reported.16 Conflicting data exist on the use of statin in previous studies, and some factors like the method of administration and duration of exposure can influence the effect of simvastatin.
This study evaluated and compared the percentage of bone formation in extraction sockets treated with simvastatin and collagen versus collagen alone.
Methods
In this single-masked sex and age-stratified, randomized clinical trial, 40 patients referring to the Periodontics Department of Dental School, Shahid Sadoughi University of Medical Sciences, Yazd, were selected (Figure 1). All the patients enrolled in this study underwent the extraction of hopeless teeth and were divided into treatment and control groups. The present study was conducted using the principles of the Helsinki Declaration and Consort Guideline 2010 (Supplementary file 1). The study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences (IR.SSU.REC.1397.120) and was registered in the IRCT registry with the identification code IRCT20171015036782N6.
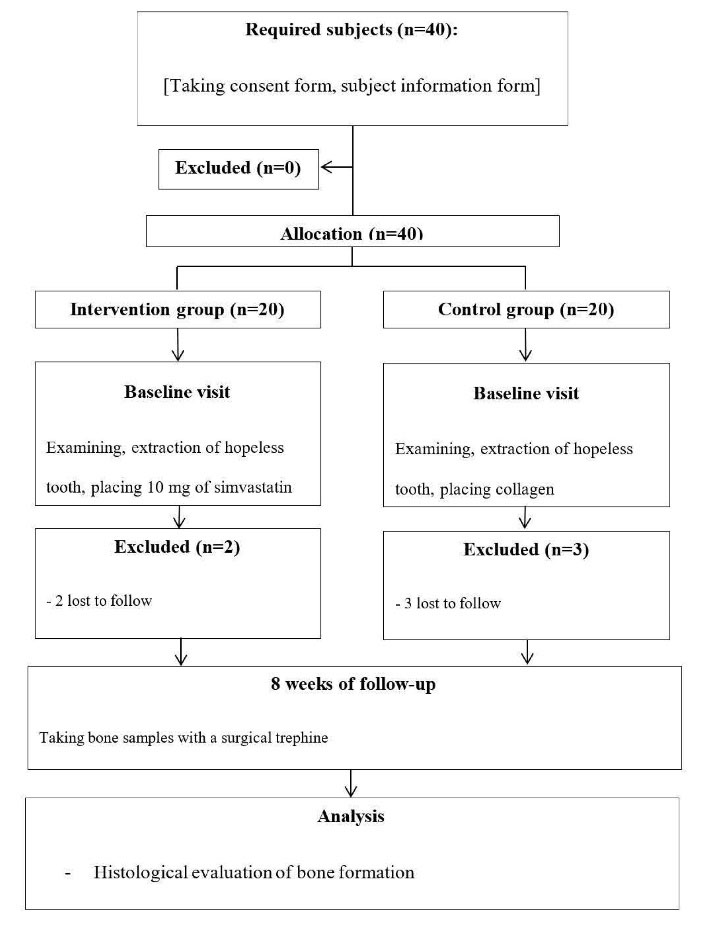
Figure 1.
Flow diagram of patient selection
.
Flow diagram of patient selection
Inclusion criteria
Patients with hopeless premolar and molar teeth and candidates for implant placement were included.
Exclusion criteria
Patients with periodontitis, systemic diseases like diabetes, pregnancy, a history of radiotherapy and steroid drugs, smoking, and a history of the systemic use of statins were excluded.
Intervention
Patients were divided into treatment and control groups according to the randomized number table by an assistant blinded to the details. The surgeon was aware of group allocation, but patients and the pathologist were blinded to group assignment. Before surgery, mouth rinsing was performed with 0.2% chlorhexidine gluconate mouthwash for 1 minute. After local infiltration anesthesia with 2% lidocaine and 1:100,000 epinephrine, a full-thickness mucoperiosteal flap was elevated, and a hopeless tooth was extracted. The intact dental socket wall was curetted and rinsed with a normal saline solution. In the treatment group, 10 mg of simvastatin (one 10-mg tablet in powdered form) in combination with collagen was placed in the extraction socket. In contrast, only collagen was placed in the control group. The socket was covered with 10*10-mm acellular dermal allograft (Cenomembrene, Hamanand Saz Baft Tissue Regeneration Corporation, KFZ, Iran), and the flap was closed with 3-0 vicryl suture to achieve primary closure. The next session was scheduled two months after extraction, in which bone samples were taken using a 3.5-mm surgical trephine from the middle part of the socket for the histologic examination. The bone samples were fixed in 10% formalin solution for 48 hours and decalcified in formic acid for one week. Histologic longitudinal sections measuring 5 µm were stained with hematoxylin and eosin (H&E). In each sample, 5 fields with the highest bone density were selected under × 400 magnification, and the image was taken with a camera attached to a microscope. ImageJ software was used to examine the images.
Additionally, foreign body reaction, inflammatory reaction, and histological features of the bone substitute material were evaluated. Figure 2 shows histological sections of osteogenesis.
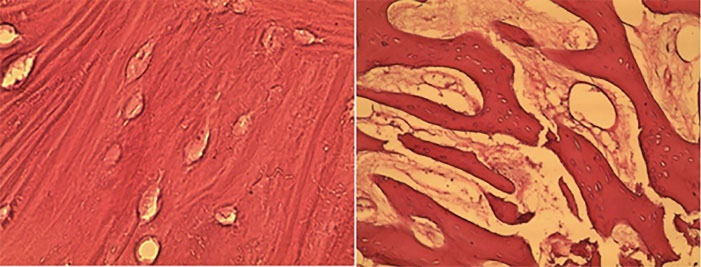
Figure 2.
Osteogenesis in the treatment and control groups at 8 weeks; left side: treatment group, right side: control group (HE × 400). Arrowheads: bone trabecula
.
Osteogenesis in the treatment and control groups at 8 weeks; left side: treatment group, right side: control group (HE × 400). Arrowheads: bone trabecula
Primary and secondary outcomes
The primary outcome of this study was the amount of bone formation, which was measured as the percentage of the bony tissue area in the total tissue area. The secondary outcomes were the percentage of inflammatory reactions, remaining bone substitutes, and foreign body reactions in the total tissue area.
Data collection method
The collected data included bone formation, foreign body reaction, a remnant of a bone substitute, and inflammatory reaction. The formation of new immature bone was calculated as a percentage of surface area in the histologic section. The foreign body reaction, defined as granulomatous inflammation and the formation of foreign body granuloma, epithelioid macrophages, and multinucleated giant cells, can be seen in histopathological examination with H&E staining. Residual graft materials were seen as amorphous material in the histologic section. The inflammatory reaction was evaluated as lymphocyte infiltration in each section.
Sample size calculation
Considering a significance level of 5%, a power of 80%, and according to the results of a previous study,22 to achieve a significant difference of at least one unit in the mean amorphous bone while anticipating a standard deviation of S = 0.6, 20 subjects were included in each group.
SPSS 23 was used for statistical analysis.
Statistical analysis
Data were measured as mean ± standard deviation and evaluated via the Kolmogorov-Smirnov test to assess normal distribution. Normally distributed data were compared via t-test, while non-normally distributed data were compared via Mann–Whitney and Fisher’s exact tests. The statistical significance level was considered at P < 0.05.
Results
Baseline characteristics
Of the 40 patients enrolled in the study, 35 completed this research. The mean age was 42.50 ± 14.52 years in the treatment group and 34.47 ± 15.02 years in the control group. The treatment group consisted of 13 males and 5 females, with 7 males and 10 females in the control group. Eighteen dental sockets were treated with simvastatin and 17 without it. There was no significant difference in age and sex between the two groups (Table 1).
Table 1.
Patient’s demographic characteristics in the treatment and control groups
|
Demographic characteristics
|
Treatment group
|
Control group
|
P
value
|
| Number, n% |
18 |
17 |
|
| Male, n% |
13 (72.2%) |
7 (41.2%) |
0.64 |
| Female, n% |
5 (27.8%) |
10 (58.8%) |
0.64 |
| Age/year |
42.50 ± 14.52 |
34.47 ± 15.02 |
0.169 |
Pearson’s chi-squared test
Primary outcome
The mean amounts of bone formation in the treatment and control groups are presented in Table 2. According to Figure 2, bone formation in the treatment group was significantly higher than in the control group. Multiple linear regression was used to eliminate the confounding factors of age and sex. These analyses showed that after age and sex matching, there was a statistically significant difference between the treatment and control groups.
Table 2.
The mean percentages of bone formation
|
Group
|
Number
|
Mean percentage of bone formation
|
SD
|
P
value
|
| Treatment |
18 |
69.28 |
3.93 |
0.0001 |
| Control |
17 |
52.76 |
2.01 |
Secondary outcome
Neither the treatment nor the control group exhibited a foreign body reaction and bone substitute remnant. Therefore, the two groups had no significant difference in histological characteristics of foreign body reaction and bone substitute remnants.
No inflammatory cell infiltration was observed in both groups, except that 23.5% of the control group showed mild chronic inflammatory reactions.
Discussion
Alveolar ridge preservation, synonymous with socket preservation, was first described as bone maintenance in 1982.23 The shape and volume of the alveolar process are determined by the presence or absence of teeth and their inclination in the bone.1,24 According to controversy regarding material choice in socket augmentation, decision-making on selecting materials in socket grafting is important. As we have limited donor sites for autogenous bone harvesting and its associated morbidity, several studies recommended using an alternative material as a substitute for autogenous bone.23 Given statins’ antibacterial, anti-inflammatory, and osteopromoting properties, their topical use is recommended as adjunctive therapy to surgical and nonsurgical periodontal treatments.25,26 Given the importance of bone preservation during tooth extraction and the reduction in bone resorption after tooth extraction, the specific aim of this study was to evaluate the effect of simvastatin on bone regeneration in human dental sockets after tooth extraction, which was defined as the proportion of newly formed bone.
In the present study, a comparison between the two groups showed that the rate of bone formation was higher in the collagen and simvastatin group compared to the other group, consistent with previous studies.19,22,27 Wu et al’s27 study indicated the effectiveness of preserving the alveolar bone of the dental socket after the topical use of simvastatin. Unlike the present study, the study above was an animal study and cannot be reliably generalized to humans. Additionally, in the study above, polylactide-co-glycolide acted as the carrier for simvastatin, unlike the collagen in our study. The follow-up duration was two months, and the treatment and control groups differed in both studies.
Rao et al19 performed a radiographic evaluation and determined clinical parameters after local delivery of simvastatin in smokers with chronic periodontitis. In this study, clinical parameters such as probing depth and clinical attachment loss were evaluated in 6- and 9-month follow-ups. They injected simvastatin gel into the periodontal pockets that had vertical bone defects. The simvastatin group sites achieved significantly greater vertical defect fill compared to the placebo group. Their methods were completely different from the present study in that the simvastatin application was different from the present study. After two months, the bone quality and quantity of the dental socket were assessed histologically, and smokers were excluded. However, both studies are valuable as they were performed on human subjects. Tanabe et al.28 showed fluvastatin’s potential for bone regeneration in an animal study. Unlike the present study, the above study was conducted outside the oral environment. Yaghobee et al21 evaluated the efficacy of simvastatin administration with bovine bone material to augment the human maxillary sinus in a split-mouth design. This study showed that the amount of newly formed bone and residual particles did not differ significantly between the two groups, even though the surgical site was the maxillary sinus and the follow-up period was 9 months. Diniz et al29 studied the effect of the local application of simvastatin (10 mg) on bone regeneration after surgical removal of bilaterally impacted mandibular third molars. The radiographic results favored simvastatin, indicating that local application of simvastatin could be a cost-effective and simple way to accelerate osseous regeneration. Koç et al30 evaluated the combination of melatonin and simvastatin on bone regeneration in rats. They demonstrated that a combination of melatonin and simvastatin had a synergistic effect on bone regeneration. The methods used in the present study were similar to those of Sezavar et al22 However, the differences are that our study’s design was not split-mouth, and the treatment and control groups had different and separate models, which are the limitations of our study. The different dosages of simvastatin in both studies are noticeable. Also, our study evaluated the presence or absence of foreign body reactions and the amount of residual graft materials. Only 23.5% of the control group subjects in our study showed an inflammatory reaction.
Contrary to our study, the samples of some studies were animal models.27,28,31 Histological evaluations were carried out in the present study in contrast to radiographic and clinical evaluations in other studies.20,31-33 Histological evaluations of the present study could assess bone quality and quantity more accurately. Another limitation of our study was the flap reflection, which can influence bone resorption. It should be noted that this procedure was done in both the treatment and control groups, and both groups were influenced by it.
Conclusion
In conclusion, the present study’s findings showed that simvastatin use in tooth sockets resulted in higher bone formation compared to the healing of the tooth socket with collagen alone. Therefore, it can be an effective substance during the healing period in tooth sockets after extraction to gain more mineralized bone.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
The data from the reported study are available upon request from the corresponding author.
Ethical Approval
The present study has the code of ethics IR.SSU.REC.1397.120 of Shahid Sadoughi University of Medical Science, Yazd, Iran.
Supplementary File
CONSORT 2010 checklist of information was used to include when reporting a randomized trial.
(pdf)
Acknowledgements
The authors would like to thank the Vice-Chancellor for Technology Research of Shahid Sadoughi University of Medical Sciences in Yazd for approving and financially supporting the project with code no. 6237.
References
- Maiorana C, Poli PP, Deflorian M, Testori T, Mandelli F, Nagursky H. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J Periodontal Implant Sci 2017; 47(4):194-210. doi: 10.5051/jpis.2017.47.4.194 [Crossref] [ Google Scholar]
- Keranmu D, Nuermuhanmode N, Ainiwaer A, Guli Guli, Taxifulati D, Shan W. Clinical application of concentrate growth factors combined with bone substitute in alveolar ridge preservation of anterior teeth. BMC Oral Health 2022; 22(1):54. doi: 10.1186/s12903-022-02091-8 [Crossref] [ Google Scholar]
- Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta-analysis. J Clin Periodontol 2019; 46 Suppl 21:195-223. doi: 10.1111/jcpe.13057 [Crossref] [ Google Scholar]
- Fischer KR, Solderer A, Arlt K, Heumann C, Liu CC, Schmidlin PR. Bone envelope for implant placement after alveolar ridge preservation: a systematic review and meta-analysis. Int J Implant Dent 2022; 8(1):56. doi: 10.1186/s40729-022-00453-z [Crossref] [ Google Scholar]
- Couso-Queiruga E, Mansouri CJ, Alade AA, Allareddy TV, Galindo-Moreno P, Avila-Ortiz G. Alveolar ridge preservation reduces the need for ancillary bone augmentation in the context of implant therapy. J Periodontol 2022; 93(6):847-56. doi: 10.1002/jper.22-0030 [Crossref] [ Google Scholar]
- Avila-Ortiz G, Gubler M, Romero-Bustillos M, Nicholas CL, Zimmerman MB, Barwacz CA. Efficacy of alveolar ridge preservation: a randomized controlled trial. J Dent Res 2020; 99(4):402-9. doi: 10.1177/0022034520905660 [Crossref] [ Google Scholar]
- Dam VV, Trinh HA, Rokaya D, Trinh DH. Bone augmentation for implant placement: recent advances. Int J Dent 2022; 2022:8900940. doi: 10.1155/2022/8900940 [Crossref] [ Google Scholar]
- Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials 2007; 28(6):1005-13. doi: 10.1016/j.biomaterials.2006.10.015 [Crossref] [ Google Scholar]
- Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants 1998; 13 Suppl:11-45. [ Google Scholar]
- Urban IA, Lozada JL. A prospective study of implants placed in augmented sinuses with minimal and moderate residual crestal bone: results after 1 to 5 years. Int J Oral Maxillofac Implants 2010; 25(6):1203-12. [ Google Scholar]
- Cáceres M, Romero A, Copaja M, Díaz-Araya G, Martínez J, Smith PC. Simvastatin alters fibroblastic cell responses involved in tissue repair. J Periodontal Res 2011; 46(4):456-63. doi: 10.1111/j.1600-0765.2011.01360.x [Crossref] [ Google Scholar]
- Park JB. The use of simvastatin in bone regeneration. Med Oral Patol Oral Cir Bucal 2009; 14(9):e485-8. [ Google Scholar]
- Edwards CJ, Spector TD. Statins as modulators of bone formation. Arthritis Res 2002; 4(3):151-3. doi: 10.1186/ar399 [Crossref] [ Google Scholar]
- Ayukawa Y, Yasukawa E, Moriyama Y, Ogino Y, Wada H, Atsuta I. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107(3):336-42. doi: 10.1016/j.tripleo.2008.07.013 [Crossref] [ Google Scholar]
- Jin H, Ji Y, Cui Y, Xu L, Liu H, Wang J. Simvastatin-incorporated drug delivery systems for bone regeneration. ACS Biomater Sci Eng 2021; 7(6):2177-91. doi: 10.1021/acsbiomaterials.1c00462 [Crossref] [ Google Scholar]
- Gupta S, Del Fabbro M, Chang J. The impact of simvastatin intervention on the healing of bone, soft tissue, and TMJ cartilage in dentistry: a systematic review and meta-analysis. Int J Implant Dent 2019; 5(1):17. doi: 10.1186/s40729-019-0168-4 [Crossref] [ Google Scholar]
- Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol 2010; 81(2):214-22. doi: 10.1902/jop.2009.090429 [Crossref] [ Google Scholar]
- Pradeep AR, Priyanka N, Kalra N, Naik SB, Singh SP, Martande S. Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with class II furcation defects: a randomized controlled clinical trial. J Periodontol 2012; 83(12):1472-9. doi: 10.1902/jop.2012.110716 [Crossref] [ Google Scholar]
- Rao NS, Pradeep AR, Bajaj P, Kumari M, Naik SB. Simvastatin local drug delivery in smokers with chronic periodontitis: a randomized controlled clinical trial. Aust Dent J 2013; 58(2):156-62. doi: 10.1111/adj.12042 [Crossref] [ Google Scholar]
- Pradeep AR, Rao NS, Bajaj P, Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol 2013; 84(1):24-31. doi: 10.1902/jop.2012.110721 [Crossref] [ Google Scholar]
- Yaghobee S, Panjnoush M, Chokami Rafiei S, Amini Shakib P, Mahmoodi S, Rasouli-Ghahroudi AA. Effect of simvastatin on bone regeneration: a histologic and histomorphometric analysis. J Oral Maxillofac Surg 2020; 78(6):927-34. doi: 10.1016/j.joms.2020.01.016 [Crossref] [ Google Scholar]
- Sezavar M, Bohlouli B, Farhadi S, Tabatabaee S, Latifi R. Simvastatin effects on dental socket quality: a comparative study. Contemp Clin Dent 2018; 9(1):55-9. doi: 10.4103/ccd.ccd_719_17 [Crossref] [ Google Scholar]
- Kalsi AS, Kalsi JS, Bassi S. Alveolar ridge preservation: why, when and how. Br Dent J 2019; 227(4):264-74. doi: 10.1038/s41415-019-0647-2 [Crossref] [ Google Scholar]
- Alenazi A, Alotaibi AA, Aljaeidi Y, Alqhtani NR. The need for socket preservation: a systematic review. J Med Life 2022; 15(3):309-12. doi: 10.25122/jml-2021-0308 [Crossref] [ Google Scholar]
- Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. Statins in nonsurgical and surgical periodontal therapy. A systematic review and meta-analysis of preclinical in vivo trials. J Periodontal Res 2018; 53(3):267-87. doi: 10.1111/jre.12514 [Crossref] [ Google Scholar]
- Akram Z, Vohra F, Javed F. Efficacy of statin delivery as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a meta-analysis. J Investig Clin Dent 2018; 9(2):e12304. doi: 10.1111/jicd.12304 [Crossref] [ Google Scholar]
- Wu Z, Liu C, Zang G, Sun H. The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg 2008; 37(2):170-6. doi: 10.1016/j.ijom.2007.06.018 [Crossref] [ Google Scholar]
- Tanabe K, Nomoto H, Okumori N, Miura T, Yoshinari M. Osteogenic effect of fluvastatin combined with biodegradable gelatin-hydrogel. Dent Mater J 2012; 31(3):489-93. doi: 10.4012/dmj.2012-008 [Crossref] [ Google Scholar]
- Diniz JA, da Silva Barbirato D, do Nascimento EH, dos Anjos Pontual A, Dourado A, Laureano Filho JR. Tomographic evaluation of the effect of simvastatin topical use on alveolar bone microarchitecture, pain and swelling after mandibular third molar extraction: a randomized controlled trial. Clin Oral Investig 2022; 26(4):3533-45. doi: 10.1007/s00784-021-04322-8 [Crossref] [ Google Scholar]
- Koç O, Tüz HH, Ocak M, Bilecenoğlu B, Fırat A, Kaymaz FF. Can the combination of simvastatin and melatonin create a synergistic effect on bone regeneration?. J Oral Maxillofac Surg 2021; 79(8):1672-82. doi: 10.1016/j.joms.2020.12.044 [Crossref] [ Google Scholar]
- Houshmand B, Hassanzadeh R, Eslami B, Amouei S, Dashti G, Morad G. Simvastatin and lovastatin induce ectopic bone formation in rat subcutaneous tissue. J Periodontol Implant Dent 2010; 2(1):12-6. [ Google Scholar]
- Saifi AM, Giraddi GB, Ahmed N. Healing of extraction socket following local application of simvastatin: a split mouth prospective study. J Oral Biol Craniofac Res 2017; 7(2):106-12. doi: 10.1016/j.jobcr.2017.04.001 [Crossref] [ Google Scholar]
- Degala S, Bathija NA. Evaluation of the efficacy of simvastatin in bone regeneration after surgical removal of bilaterally impacted third molars-a split-mouth randomized clinical trial. J Oral Maxillofac Surg 2018; 76(9):1847-58. doi: 10.1016/j.joms.2018.04.035 [Crossref] [ Google Scholar]