J Adv Periodontol Implant Dent. 16(1):44-48.
doi: 10.34172/japid.2024.006
Research Article
Effect of conventional cigarettes and e-cigarettes on salivary biomarkers: A systematic review
Amirmohammad Dolatabadi Conceptualization, Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing, 1 
Faranak Noori Validation, Visualization, Writing – review & editing, 2 
Amir Raee Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Software, Supervision, Writing – review & editing, 1, * 
Author information:
1Department of Periodontology, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
2Department of Endodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background.
E-cigarette consumption is increasing, and like conventional smoking, it can cause some harmful effects. This systematic review compared the effect of conventional cigarettes and e-cigarettes on salivary biomarkers.
Methods.
The search strategies included electronic databases (Medline/PubMed, Scopus, EMBASE) and related journals up to May 2023. A qualitative assessment was performed on data extracted from the included studies. Seven studies were included in this systematic review (number of patients=563).
Results.
Eleven biomarkers were assessed and compared between e-cigarette and conventional cigarette smokers. The data showed that the different effects of electronic and conventional cigarettes on the level of these biomarkers were not achievable. Due to the heterogeneity of the studies (I2 statistic>90%), performing a meta-analysis was impossible. Even after a sub-group classification, homogeneous data were not achieved.
Conclusion.
The current data do not provide evidence of obtainable outcomes between conventional cigarettes and e-cigarettes on salivary biomarkers.
Keywords: Cytokines, Smokers, Tobacco products
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Smoking has harmful effects on human health that have been discussed in several studies.1 Traditional tobacco products (e.g., smoking combustible cigarettes) can increase the risk of severe disorders like cancer and cardiopulmonary and metabolic diseases.2 Smoking also has significant adverse effects on oral health, with relationships between smoking and periodontal diseases, wound healing, and oral cancers.1
Electronic cigarettes have become popular, with over two million Britons now regularly vaping.3 Despite the existence of the idea that e-cig vaping is safer than cigarette smoking, many epidemiological studies have shown its adverse effects.4 In e-cigarettes, nicotine is provided for inhalation by heating a solution that contains water, nicotine, propylene glycol, and vegetable glycerin.3 Recent studies have shown that e-cigs can change heart rate, blood pressure, and other vital signs and symptoms. Smoking e-cigarettes can increase neutrophil activation and change mucin secretion. Because of the exposure to harmful organic and inorganic compounds (including metals), e-cigarette users are more susceptible to developing cancer than nonusers.4
It has been reported that periodontal status, plaque index (PI), clinical attachment loss (CAL), probing depth (PD), and marginal bone loss are worse in individuals using e-cigarettes and other electronic nicotine delivery systems (ENDS) than in the controls (individuals who have never used tobacco in any form).5
Different biofluids, such as blood, gingival crevicular fluid, and saliva, have been used for their diagnostic or prognostic value for disease detection.6 Considering its advantages, such as ease and noninvasive collection, saliva can be a potential alternative to blood tests. Also, in many studies, saliva has been used as a target vehicle for different biomarkers in oral diseases.7
Various salivary biomarkers can play important roles in oral health status. For instance, interleukin-6 (IL-6) can activate osteoclast formation and facilitate bone resorption and T-cell differentiation. In addition, IL-6 is implicated in periodontitis. Another crucial biomarker to indicate is the IL-8, which is involved in the selective recruitment and activation of neutrophils.3 In addition, the existence of many biomarkers in saliva causes a benefit in diagnostic and prognostic issues. For instance, salivary levels of tumor necrosis factor α (TNF-α), IL-1, IL-4, IL-6, and IL-8 have been described as relevant biomarkers for oral lichen planus diagnosis and prognosis.8 Also, IL-1β, TNF-α, IL-6, and the receptor activator of nuclear factor κB ligand (RANKL), among other cytokines, are known to be involved in immune response regulation in periodontal diseases.9
Due to the abovementioned features of saliva, it is a favorable oral fluid to determine the health status of the oral cavity, including the presence of periodontal diseases.10
To the best of our knowledge, no systematic review has been conducted on the in vivo effects of conventional cigarettes and e-cigarettes on salivary biomarkers. Therefore, the current study compared the effects of conventional cigarettes and e-cigarettes on salivary biomarkers.
Methods
The present systematic review was conducted according to PRISMA statement guidelines.11 The protocol of this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42023440189. The question focused on in this study was: “What is the comparative effect of conventional cigarettes and e-cigarettes on salivary biomarkers?” The differences in salivary biomarkers in conventional and e-cigarette smokers were considered the primary outcome of this systematic review.
This question has been articulated as follows:
-
Population: electronic and conventional cigarette smokers
-
Intervention: conventional cigarette smokers
-
Comparison: electronic cigarette smoking
-
Outcomes: salivary biomarkers
Search strategy
We systematically reviewed the literature within three main electronic databases (Medline/PubMed, Scopus, and EMBASE) to identify all articles comparing salivary biomarkers between conventional and e-cigarette smokers up to May 2023. We also searched cross-references to complement the evidence given in this review. The literature was searched using the electronic search strategy (Supplementary file 1).
The present review included case-control and cross-sectional studies that compared salivary biomarkers in conventional and e-cigarette smokers. Retrospective studies, case series, case reports, animal studies, in vitro studies, letters, conference abstracts, and brief reports were excluded.
Study selection
Two authors (AD and AR) independently screened the titles (and abstracts, if necessary) of the studies to determine the articles that met the inclusion criteria. If there was any conflict, a third reviewer (FN) made a judgment. All full texts of the studies meeting the inclusion criteria were assessed for quality.
Quality assessment
Two reviewers (AD and AR) independently assessed the quality of the included studies. For each study, the risk of bias was assessed using The Joanna Briggs Institute’s Risk of Bias tool. The tool comprises eight items (clarity of criteria for inclusion, description of e the study subjects and the setting, validity and reliability of exposure measurement, using objective, standard criteria for measurement of the condition, identification of confounding factors, strategies to deal with confounding factors, and validity and reliability of outcomes measurement, using appropriate statistical analysis). Assessing bias led to the judgment of low risk of bias if all the domains were evaluated as low risk of bias, unclear risk of bias if at least one item was assessed as unclear risk of bias, or high risk of bias if at least one item was rated as high risk of bias. Any disagreement was resolved by discussion with a third reviewer (FN) to reach a consensus (Table 1).
Table 1.
Quality assessment of included studies
|
Study
|
Were the criteria for inclusion in the sample clearly defined?
|
Were the study subjects and the setting described in detail?
|
Was the exposure measured in a valid and reliable way?
|
Were objective, standard criteria used for measurement of the condition?
|
Were confounding factors identified?
|
Were strategies to deal with confounding factors stated?
|
Were the outcomes measured in a valid and reliable way?
|
Was appropriate statistical analysis used?
|
| Ye et al,12 2018 |
Yes |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
| Mokeem et al,13 2018 |
Yes |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
| Verma et al,15 2021 |
Yes |
Yes |
Yes |
Yes |
No |
No |
Yes |
Unclear |
| Faridoun et al,17 2021 |
No |
Yes |
Yes |
Yes |
No |
Unclear |
Yes |
Yes |
| Ali et al,5 2022 |
Yes |
Yes |
Yes |
Yes |
YES |
Unclear |
Yes |
Yes |
| Kamal et al,14 2022 |
Yes |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
| Miluna et al,16 2022 |
Yes |
Yes |
Yes |
Yes |
No |
Unclear |
Yes |
Yes |
Data analysis
The biomarkers’ level as a continuous outcome was presented as mean differences. All the outcomes were reported with their associated 95% confidence interval and analyzed in RevMan version 5.4 according to a random-effects model using the inverse-variance method for continuous outcomes. The heterogeneity of effects was evaluated using Higgins’ I2 statistic.
Results
Study selection
The search yielded 286 articles: 198 obtained via PubMed, 25 via Embase, 63 via Scopus, and 0 via hand research. After removing duplicates, 239 records were screened for titles and abstracts, and 206 studies were excluded due to not meeting the inclusion criteria, leaving 33 articles for full-text assessment. After a full-text review, 26 articles were excluded for the following reasons: not assessing salivary biomarkers, lack of comparison between e-cigarettes and conventional cigarettes, and being prospective cohort studies. Therefore, seven studies, all of which were case-control and cross-sectional, were included in this systematic review and used for the qualitative and quantitative analyses (see Figure 1).
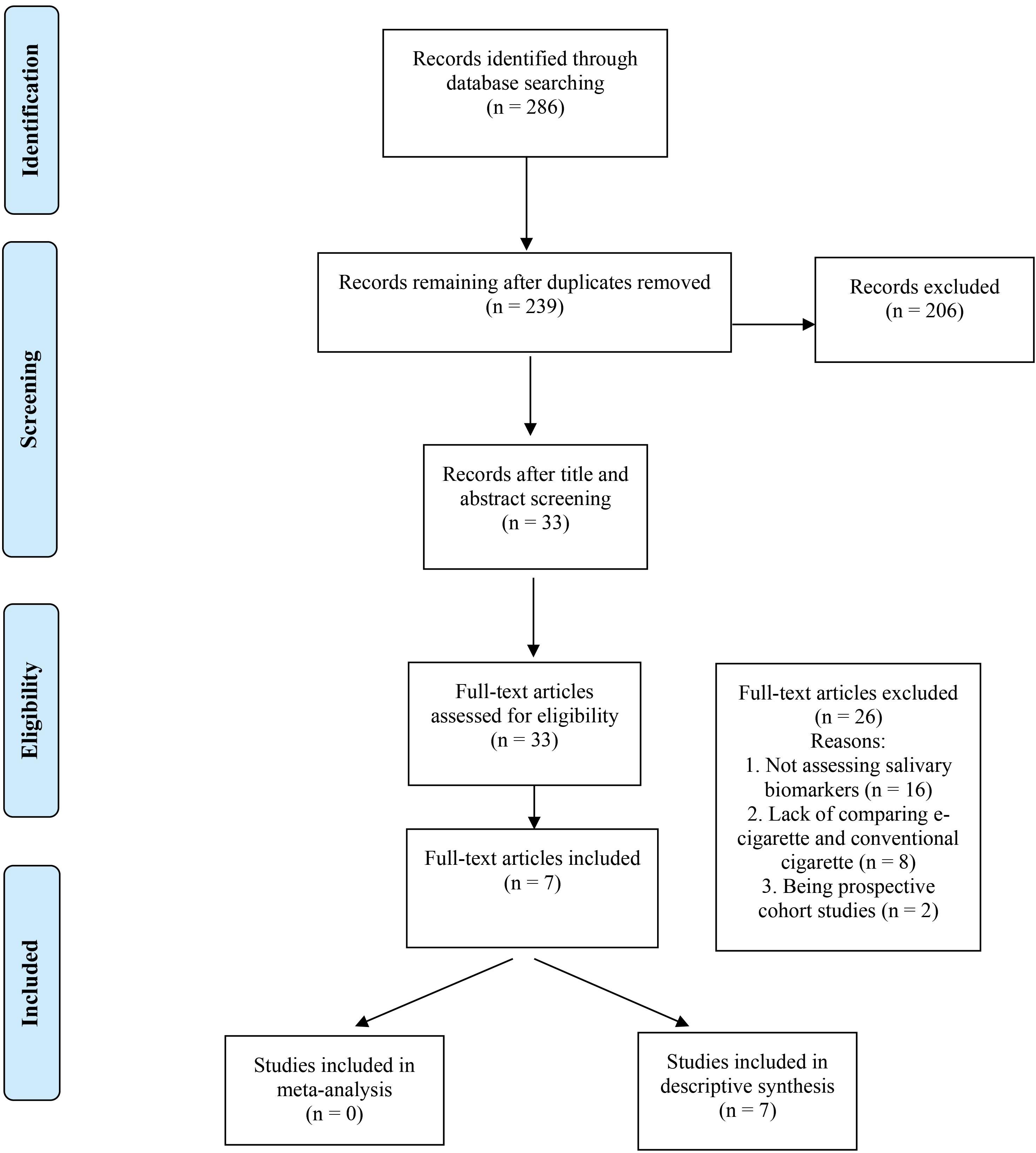
Figure 1.
Flowchart of the Search strategy
.
Flowchart of the Search strategy
Table 2 summarizes the characteristics of the studies included in this systematic review.
Table 2.
Characteristics of the included studies
|
Study
|
Study design
|
Outcome measures
|
Study groups
|
Population
|
Duration of smoking
|
| Verma et al15 |
Cross-sectional |
IL1β, IL6, IL8, IL10, IL1RA, CRP, TNFα |
e-cigarette smoker - conventional smoker - both smoker - nonsmoker |
38 Males
22 Females |
Not mentioned |
| Ye et al12 |
Cross-sectional |
PGE-2, IL-1β |
e-cigarette smoker - conventional smoker - both smoker - nonsmoker |
24 Males
24 Females |
Not mentioned |
| Miluna et al16 |
Cross-sectional |
IL-6, IL-1β, IL-8, TNFα |
Snus - Cigarettes - E-cigarettes - nonsmoker |
38 Males
38 Females |
Not mentioned |
| Mokeem et al13 |
Cross-sectional |
IL-1β, IL-6 |
cigarette-smokers, waterpipe-smokers, E-cig users - never-smokers |
154 Males |
Cigarette: 16.2 ± 2.5 per day
e-Cigarette: 9.2 ± 1.4 per day |
| Faridoun et al17 |
Cross-sectional |
IL1β, IL6, IL8, IL10, IL1RA, CRP, TNFα |
Conventional cigarettes - E-cigarettes - Mixed use - No smoking |
37 Males
27 Females |
Not mentioned |
| Ali et al5 |
Cross-sectional |
IL-15, IL-18 |
Current cigarette smokers - ENDS users - Never-smokers with periodontitis - Never-smokers without periodontitis |
54 Males
21 Females |
Cigarette: 24.3 ± 0.7 pack years
e-Cigarette: 12.5 ± 0.8 years |
| Kamal et al14 |
Cross-sectional |
IL1β, TGFβ |
e-cigarette smoker - conventional smoker - nonsmoker |
86 Males |
Cigarette: 14.7 ± 2.5 per day
e-Cigarette: 10.1 ± 1.4 per day |
General characteristics of the included studies
The outcomes of the studies are presented in Supplementary file 2. The included studies were published between 2018 and 2022, and the number of patients enrolled in the studies ranged between 24 and 100. The total number of patients who participated in the seven studies was 563, with 431 men and 132 women.
Salivary biomarkers
In total, eleven biomarkers were assessed in seven case-control studies. The measured salivary biomarkers were IL-1β, IL-6, IL-8, IL-10, IL-1RA, CRP, TNF-α, PGE2, IL-15, IL-18, and TGF-β. Salivary IL-1β levels were measured in 6 studies. In three studies,12-14 it was higher in conventional smokers, and in others,15-17 e-cigarette users had higher levels of IL-1β. IL-6 biomarker was assessed in four studies, and all of them except one,16 reported higher levels in conventional smokers. Also, three studies evaluated IL-8 and15-17 showed higher IL-8 biomarker levels in conventional smokers, and in one study,16 it was vice versa.
TGFβ and PGE2 levels were only reported in one study,12,14 and both these biomarkers were higher in conventional smokers. Two studies15,17 reported that CRP and IL-1RA levels in conventional smokers were higher than those in e-cigarette users, with higher IL-10 biomarker salivary levels in e-cigarette users. Regarding TNFα, two studies showed higher salivary levels in conventional smokers15,17 and one study reported vice versa.16 Finally, IL-15 and IL-18 salivary levels were assessed in one study5; this biomarker’s level was higher in conventional smokers.
Due to the heterogeneity of the studies (I2 statistic > 90%), performing a meta-analysis was impossible. Even after a sub-group classification, homogenous data were not achieved.
Discussion
This systematic review compared the effect of conventional cigarettes and e-cigarettes on salivary biomarkers. Seven studies were finally included in this systematic review, and all were cross-sectional. The salivary biomarkers that were assessed showed different values between conventional and e-cigarette smokers.
Levels of pro-inflammatory biomarkers, including IL-1β, IL-6, IL-8, CRP, TNF-α, IL-15, and IL-18, and anti-inflammatory biomarkers like TGF-β, IL-10, IL-1RA, and PGE2 were assessed in the included studies.
Flieger et al18 investigated the levels of thiocyanate in the saliva of tobacco smokers in comparison to e-cigarette smokers and nonsmokers. Salivary thiocyanate is responsible for various neurological disorders (amblyopia, infant squint in children of smoking mothers) and endocrine diseases (an increase in the frequency of nodular goiter). They reported that the salivary thiocyanate levels in e-cigarette smokers were not significantly different from tobacco smokers but higher compared to nonsmokers. This finding suggests that e-cigarettes may not be as harmful as they were thought.
Akiyama and Sherwood,19 in their systematic review in 2021 on changes in tobacco-related biomarker levels, concluded that using e-cigarettes could lead to a significant reduction in exposure to harmful substances compared to combusted cigarettes. In the present study, we specifically focused on salivary biomarkers and included several newly published studies.
Interestingly, there were some conflicts in biomarkers’ measurements between studies, which made it difficult or even impossible in some cases to conclude the effect of conventional and e-cigarettes on salivary biomarkers. For instance, IL-1b was the most assessed biomarker in studies12-17 but half of them12-14 reported that its amount was higher in conventional smokers, and others showed that it was higher in e-cigarette users’ saliva. We hypothesize that these differences stem from heterogeneous methods in different studies. There were some critical differences in the survey of reasons why the outcomes of studies are not comparable. For instance, the use of antibiotics was not mentioned in the exclusion criteria in one study,17 while antibiotics might interfere with the quantity and quality of salivary biomarkers. Another issue was the different gender distribution in studies. There were two studies13,14 with only male participants. Another reason is that the time of cigarette and e-cigarette consumption in studies was not similar; thus, different exposure times might have led to various outcomes.
Wadia et al3 assessed inflammatory cytokines (IL-1β and IL-8) in a group of established smokers before and after substituting vaping for smoking tobacco. They claimed that no definitive conclusions could be drawn from this dataset due to the limited sample size and large variations. Also, due to the study design of switching from tobacco smoking to vaping in participants, the results could be misinterpreted.
Conclusion
In this study, we could not agree on the different effects of conventional cigarettes and e-cigarettes on salivary biomarkers due to the heterogeneity of the included studies. We suggest that future studies use a standard method to enable more conclusive analyses.
Competing Interests
The authors declare that they have no financial and non-financial competing interests with regard to the publication of their work during submission.
Consent for Publication
Not applicable.
Data Availability Statement
All the available data have been included in the submitted files, and Additional data are available in Supplementary file 1.
(pdf)
All the available data have been included in the submitted files, and Additional data are available in Supplementary file 2.
(pdf)
Ethical Approval
Not applicable.
Funding
No funding.
References
- Holliday RS, Campbell J, Preshaw PM. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells A systematic review of the literature. J Dent 2019; 86:81-8. doi: 10.1016/j.jdent.2019.05.030 [Crossref] [ Google Scholar]
- Alqahtani S, Cooper B, Spears CA, Wright C, Shannahan J. Electronic nicotine delivery system-induced alterations in oral health via saliva assessment. Exp Biol Med (Maywood) 2020; 245(15):1319-25. doi: 10.1177/1535370220941258 [Crossref] [ Google Scholar]
- Wadia R, Booth V, Yap HF, Moyes DL. A pilot study of the gingival response when smokers switch from smoking to vaping. Br Dent J 2016; 221(11):722-6. doi: 10.1038/sj.bdj.2016.914 [Crossref] [ Google Scholar]
- Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res 2019; 5(4):00182-2019. doi: 10.1183/23120541.00182-2019 [Crossref] [ Google Scholar]
- Ali D, Kuyunov I, Baskaradoss JK, Mikami T. Comparison of periodontal status and salivary IL-15 and -18 levels in cigarette-smokers and individuals using electronic nicotine delivery systems. BMC Oral Health 2022; 22(1):655. doi: 10.1186/s12903-022-02700-6 [Crossref] [ Google Scholar]
- Khan ZM, Waheed H, Khurshid Z, Zafar MS, Moin SF, Alam MK. Differentially expressed salivary proteins in dental caries patients. Biomed Res Int 2021; 2021:5517521. doi: 10.1155/2021/5517521 [Crossref] [ Google Scholar]
- Paqué PN, Hjerppe J, Zuercher AN, Jung RE, Joda T. Salivary biomarkers as key to monitor personalized oral healthcare and precision dentistry: a scoping review. Front Oral Health 2022; 3:1003679. doi: 10.3389/froh.2022.1003679 [Crossref] [ Google Scholar]
- Humberto JS, Pavanin JV, da Rocha MJ, Motta AC. Cytokines, cortisol, and nitric oxide as salivary biomarkers in oral lichen planus: a systematic review. Braz Oral Res 2018; 32:e82. doi: 10.1590/1807-3107bor-2018.vol32.0082 [Crossref] [ Google Scholar]
- Melguizo-Rodríguez L, Costela-Ruiz VJ, Manzano-Moreno FJ, Ruiz C, Illescas-Montes R. Salivary biomarkers and their application in the diagnosis and monitoring of the most common oral pathologies. Int J Mol Sci 2020; 21(14):5173. doi: 10.3390/ijms21145173 [Crossref] [ Google Scholar]
- Bostanci N, Mitsakakis K, Afacan B, Bao K, Johannsen B, Baumgartner D. Validation and verification of predictive salivary biomarkers for oral health. Sci Rep 2021; 11(1):6406. doi: 10.1038/s41598-021-85120-w [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [Crossref] [ Google Scholar]
- Ye D, Gajendra S, Lawyer G, Jadeja N, Pishey D, Pathagunti S. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: a pilot study. J Periodontol 2020; 91(10):1274-83. doi: 10.1002/jper.19-0457 [Crossref] [ Google Scholar]
- Mokeem SA, Alasqah MN, Michelogiannakis D, Al-Kheraif AA, Romanos GE, Javed F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette- and waterpipe-smokers and E-cig users. Environ Toxicol Pharmacol 2018; 61:38-43. doi: 10.1016/j.etap.2018.05.016 [Crossref] [ Google Scholar]
- Kamal NM, Shams NS. The impact of tobacco smoking and electronic cigarette vaping on salivary biomarkers A comparative study. Saudi Dent J 2022; 34(5):404-9. doi: 10.1016/j.sdentj.2022.05.003 [Crossref] [ Google Scholar]
- Verma A, Anand K, Bhargava M, Kolluri A, Kumar M, Palve DH. Comparative evaluation of salivary biomarker levels in e-cigarette smokers and conventional smokers. J Pharm Bioallied Sci 2021; 13(Suppl 2):S1642-5. doi: 10.4103/jpbs.jpbs_393_21 [Crossref] [ Google Scholar]
- Miluna S, Melderis R, Briuka L, Skadins I, Broks R, Kroica J. The correlation of Swedish snus, nicotine pouches and other tobacco products with oral mucosal health and salivary biomarkers. Dent J (Basel) 2022; 10(8):154. doi: 10.3390/dj10080154 [Crossref] [ Google Scholar]
- Faridoun A, Sultan AS, Jabra-Rizk MA, Weikel D, Varlotta S, Meiller TF. Salivary biomarker profiles in e-cigarette users and conventional smokers: a cross-sectional study. Oral Dis 2021; 27(2):277-9. doi: 10.1111/odi.13533 [Crossref] [ Google Scholar]
- Flieger J, Kawka J, Tatarczak-Michalewska M. Levels of the thiocyanate in the saliva of tobacco smokers in comparison to e-cigarette smokers and nonsmokers measured by HPLC on a phosphatidylcholine column. Molecules 2019; 24(20):3790. doi: 10.3390/molecules24203790 [Crossref] [ Google Scholar]
- Akiyama Y, Sherwood N. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicol Rep 2021; 8:282-94. doi: 10.1016/j.toxrep.2021.01.014 [Crossref] [ Google Scholar]