J Adv Periodontol Implant Dent. 15(2):100-107.
doi: 10.34172/japid.2023.018
Research Article
Comparative effect of anthocyanin on proliferation and migration of human gingival fibroblasts in the absence or presence of nicotine
Sarina Azimian Funding acquisition, Writing – original draft, Writing – review & editing, 1 
Maryam Torshabi Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing, 2 
Zeinab Rezaei Esfahrood Conceptualization, Project administration, Supervision, Writing – review & editing, 3, * 
Author information:
1Department of Periodontics, Shahid Beheshti Dental School, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Dental Biomaterials, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Department of Periodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Background.
Oral fibroblast malfunction can result in periodontal diseases. Nicotine can prolong the healing process as an irritant of oral tissues. Anthocyanins have been demonstrated to have potential benefits in preventing or treating smoking-related periodontal diseases. Cyanidin chloride’s (CC’s) potential in oral wound healing and the viability, proliferation, and migration of human gingival fibroblasts (HGFs) were examined in the presence and absence of nicotine by an in vitro study.
Methods.
The effects of different nicotine concentrations (1, 2, 3, 4, and 5 mM) on the viability and proliferation of HGF cells were evaluated in the presence and absence of different CC concentrations (5, 10, 25, and 50 μM) using the quantitative MTT assay. The scratch test was performed to evaluate the migration of CC-treated cells in the presence of 2.5-mM nicotine.
Results.
No cytotoxicity was observed at 1‒100 μM CC concentrations after 24, 48, and 72 hours of exposure to HGF cells. However, a concentration of 200 μM significantly reduced cell viability by about 20% at all the three-time intervals (P<0.05). Also, 3‒5-mM concentrations of nicotine significantly reduced cell viability in a dose- and time-dependent manner. Moreover, the understudied CC concentrations decreased nicotine’s adverse effects on cell migration to some extent.
Conclusion.
Although the understudied CC concentrations could not significantly reduce the adverse effects of understudied nicotine concentrations on the viability and proliferation of HGF cells, they were able to reduce the detrimental effects of nicotine on cell migration significantly.
Keywords: Anthocyanins, Fibroblasts, Migration, Nicotine, Proliferation
Copyright and License Information
© 2023 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral wound healing is a multi-phased process that includes cell proliferation and migration, collagen remodeling and deposition, wound contraction, and angiogenesis.1,2 Successful oral wound healing requires the complex coordination of various cell types, including fibroblasts, endothelial cells, macrophages, keratinocytes, and platelets.2 Human gingival fibroblasts (HGFs) are abundant cells in gingival connective tissue.3 Due to the role and importance of these cells, their damage results in gingival breakdown.2
Several factors damage the oral mucosa, one of the most important of which is smoking. Smoking is known as an important risk factor for noncommunicable chronic diseases.4 Various in vitro studies have shown that nicotine and smoking can impair the proliferation, migration, and attachment of gingival fibroblasts.5
Cigarette nicotine reduces oxygen supply to gingival tissues by decreasing gingival blood flow and the number of circulating cells.6,7 It reduces fibroblasts’ collagen and non-collagen protein production, negatively affecting oral fibroblasts’ viability, migration, and differentiation and causing periodontal tissue damage.8-10
Inflammation and damage to the gingival and periodontal tissues occur due to the complex interactions between periodontal pathogens and immune system inflammatory responses. Among them, reactive oxygen species (ROS) and free radicals contribute significantly to the development and progression of periodontitis.11
In some cases, periodontal treatment alone is not adequate. Although poor nutrition does not cause periodontal disease directly, many studies believe that disease progression is faster and more severe in people with poor diets due to an impaired host response.12
Antioxidants are found in all body fluids and tissues. They constitute the body’s first line of defense against free radical damage caused by cigarette smoke, medications, illness, and stress.13,14 Recent studies have shown that antioxidant supplements are essential for preventing and successfully treating disorders of gingival tissue and other tooth-supporting structures.8,15,16
Anthocyanins, a group of highly reactive antioxidants, can interact with free electrons in free radical molecules by donating an electron to oxygen species to reduce their toxic effects. Nicotine stimulates the production of free radicals, which is associated with oxidative stress and a deficient antioxidant defense mechanism. Therefore, anthocyanins may protect gingival tissues against nicotine toxicity by reducing free radicals.17
Cyanidin chloride (CyCl or CC) suppresses the RANKL-stimulated nuclear factor-kappa B (NF-κB) signaling pathway and stimulates the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. It should be noted that NF-κB and Nrf2 are the two main transcription factors involved in regulating oxidative reactions and cell proliferation.18,19
In this in vitro study, we examined the antagonistic effects of anthocyanin and CC on the toxicity of nicotine to HGFs.
Methods
Materials
The HGF1 PI 1 (NCBI: C165) cell line used in this study was obtained from the Pasture Institute Cell Bank, Tehran, Iran. The anthocyanin CC, MTT (3- [4,5-dimethylthiazol-2-yl] -2,5-diphenyl tetrazolium bromide), DMSO (dimethyl sulfoxide), and crystal violet were purchased from Sigma-Aldrich (Germany). The nicotine was obtained from MP Biomedicals (France). DMEM (Dulbecco’s Modified Eagles Medium), FBS (Fetal Bovine Serum), and antibiotics were purchased from Gibco (UK).
Cell viability assay
First, the cells were cultured with DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotic (regular medium; RM). They were incubated at 37 °C under 5% CO2 and 95% humidity. The logarithmic phase cells were then treated with different concentrations of nicotine and anthocyanin CC (in RM) separately and in combination. Untreated cells (treated with RM alone; no cytotoxicity, 100% viability) were used as negative controls for cell viability, proliferation, and migration assays.
In this study, we evaluated and compared nicotine’s cytotoxicity and its effect on the viability and proliferation of gingival fibroblasts in the presence or absence of the antioxidant anthocyanin, CC, by the MTT assay (ISO-10993-5; 2009). The nicotine concentration range used in this study was selected from earlier research.20-22 On the first day, the studied cells, which were in the logarithmic growth phase, were carefully counted and planted in each well of 96-well culture plates (3500 cells/100 µL of RM/well). The plates were then incubated in a cell culture incubator for 24 hours (37 °C, with 95% humidity and 5% CO2). On the second day of the study (50‒60% cell confluence), different concentrations of anthocyanin CC (1, 5, 10, 25, 50, 100, and 200 μM) were added to the wells separately (six identical replicates for each concentration). It should be noted that zero concentration of CC (under two conditions: RM alone and RM containing a concentration of DMSO found in the highest concentration of CC solution) was considered the control group (normal conditions of cell growth and proliferation). After 24 (for acute cytotoxicity), 48, and 72 (for chronic cytotoxicity) hours, the medium on the cells in each cell was gently and carefully drained and replaced with a culture medium (serum-free and antibiotic-free) containing 10% MTT. The plates were then incubated for 3 hours, and after confirming the formation of formazan crystals under an inverted microscope, MTT dye was drained from each well, and the same amount of DMSO solvent was added to each well. Then, the light absorption of the resulting color solutions was read using an ELISA reader. The average light absorption of each group treated with the investigated materials was divided by the average light absorption of the control group (untreated cells, non-treated, no cytotoxicity = 100% viable) and multiplied by 100 to determine the percentage of cell viability. According to the ISO-10-993-5 standard (2009), a group is considered cytotoxic if it reduces viability by more than 30% compared to the control group (viability rate falls below 70%).23
After determining the selected nicotine concentrations (concentrations of 1, 2, 3, 4, and 5 mM) obtained from previous studies,21,22 and also selecting the appropriate concentrations of anthocyanin CC obtained from the MTT test (concentrations of 0, 5, 10, 25 and 50 μM), in the second phase of the study, the possible antagonistic effects of the studied antioxidant were investigated. Thus, HGFs (3500/well) were cultured in each well of 96-well cell culture plates on the first day at a logarithmic growth phase. The cells were then treated with different concentrations of nicotine in the presence of different concentrations of anthocyanins on the second day. It should be noted that the complete, RM alone, and without nicotine and CC, was considered as the control group. The MTT test was then performed to evaluate cell viability and proliferation percentages 24 and 72 hours after cell treatment.
Cell migration assay
An in vitro scratch assay was performed to evaluate the HGF cell migration rate (which indicates the cell’s ability to repair) after stimulation with a selective concentration of nicotine (2.5 mM) and in the presence and absence of three selected concentrations (10, 25, and 50 mM) of the antioxidant anthocyanin CC.22
On the first day of the study, 100 000 HGFs in the logarithmic growth phase were cultured in each well of a 24-well plate. On the second day, when the cells had reached 100% confluence, rapid and vertical scratches were made in each well using a 100-µL sterile tip (time zero). Each well was rinsed twice with RM to remove unattached cells. The cells were then treated with the indicated nicotine and CC concentrations. The cells treated with RM alone (without nicotine and CC) were considered the control group. At zero time and 24 hours after scratching, the medium in each well was drained, and the cells were washed twice with a cold (4 °C) positive PBS buffer (containing calcium and magnesium). The cells were fixed by adding 500 µL of 100% pre-cooled methanol to each well, and the mixture was allowed to remain at room temperature (RT) for 10 minutes. For staining, methanol was drained, and 0.5% crystal violet dye solution was added (10 minutes, RT). The dye solution was then drained, and the cells were washed three times with deionized water. A digital image of the cells at the scratch site was captured under an inverted light microscope at × 4 magnification. Finally, using the ImageJ software, the distance between the two edges of the scratches in the samples was quantified (in pixels), and the migration percentage was calculated.
Statistical analysis
The results were statistically analyzed using GraphPad Prism 9 software and one-way ANOVA, followed by Tukey’s post hoc tests. A P value < 0.05 indicated statistically significant differences between the groups.
Due to the laboratory nature of the research (in vitro), the use of cells in the cell culture environment did not have any special ethical considerations.
Results
Effects of different CC concentrations on the viability and proliferation of HGFs
At 24 and 72 hours after treatment (Figure 1), there were no statistically significant differences in cell viability and proliferation between 1‒100 μM concentrations of CC and also with the control group (zero CC concentration) (P > 0.05). At a 200-μM concentration, a 20% reduction in cell viability was observed in all three study time intervals (without significant differences between them). In general, none of the 1‒100 μM concentrations caused cytotoxicity (reduction of cell viability by > 30%). On the other hand, no significant proliferation (significant increase in the number of cells over time) was observed in any concentration. Therefore, four concentrations of 5, 10, 50, and 100 M were chosen for the subsequent investigation stage.
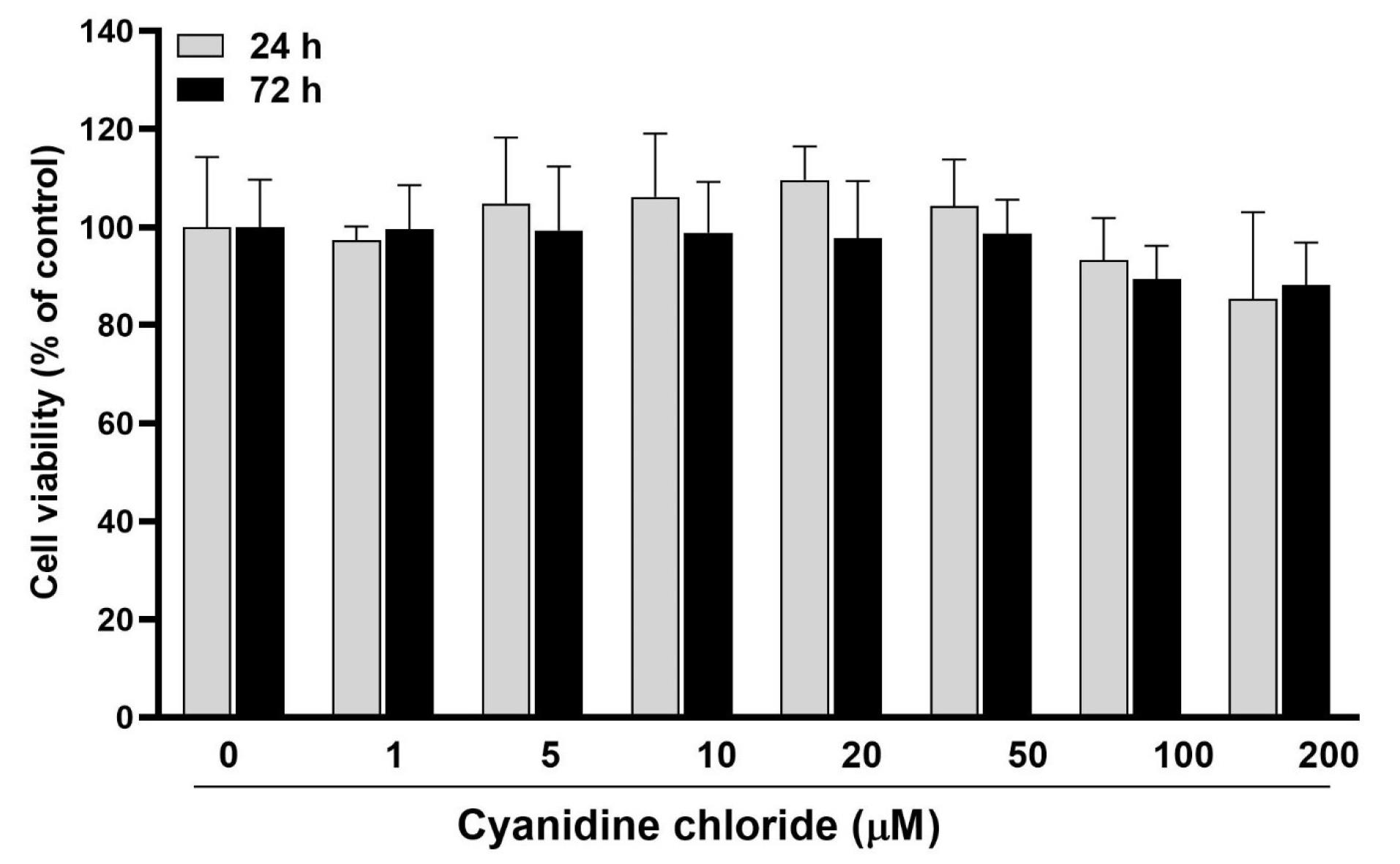
Figure 1.
The effects of different anthocyanin CC concentrations (0 to 200 μM) on the viability and proliferation of HGFs 24, 48, and 72 hours after treatment
.
The effects of different anthocyanin CC concentrations (0 to 200 μM) on the viability and proliferation of HGFs 24, 48, and 72 hours after treatment
Effects of different nicotine concentrations on the morphology, viability, and proliferation of HGFs
A qualitative microscopic observation method ( × 10 magnification) was used to study the cell morphology. The morphology of HGF cells exposed to 1- and 5-mM concentrations of nicotine for 24 hours (acute toxicity) and 72 hours (chronic toxicity) was observed (Figure 2). At 1- and 2-mM concentrations of nicotine, the morphology was not very different from the control group (zero concentration) (no acute and chronic cytotoxicity). However, at 3- and 5-mM concentrations, especially 72 hours after exposure (chronic toxicity), a change in morphology was observed: vacuolization of cytoplasm, a decrease in the number of normal cells, and an increase in the number of apoptotic cells.
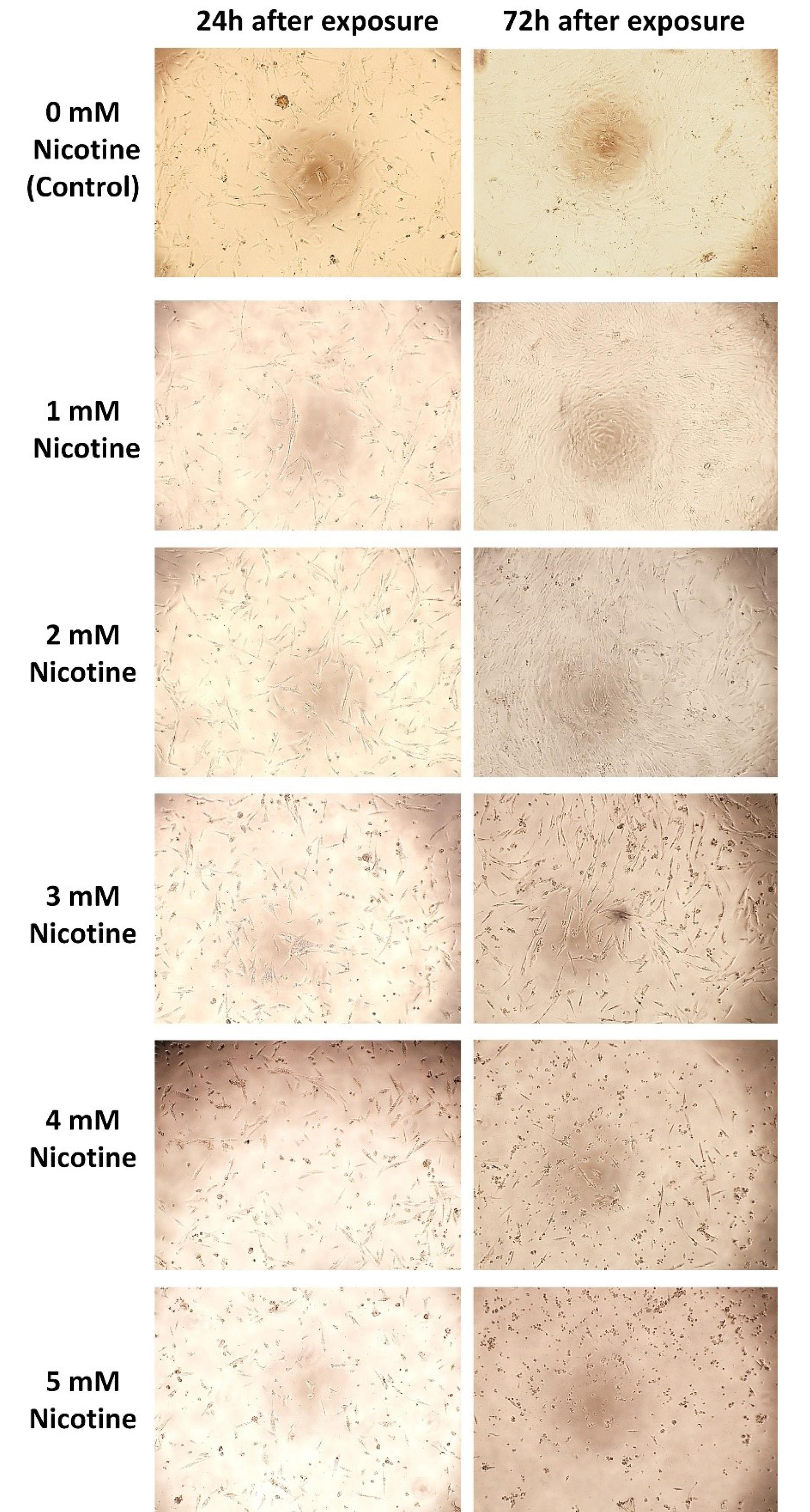
Figure 2.
Qualitative (microscopic) observation of the morphology of HGFs exposed to zero (control) to 5-mM nicotine concentrations 24 and 72 hours after exposure
.
Qualitative (microscopic) observation of the morphology of HGFs exposed to zero (control) to 5-mM nicotine concentrations 24 and 72 hours after exposure
Effects of different nicotine concentrations on the viability and proliferation of HGFs in the presence and absence of different CC concentrations
After 24 hours of exposure (Figure 3A) (obtained from the results of the quantitative MTT test), a statistically significant decrease in cell viability percentage compared to the control group (zero concentration of nicotine) was observed at concentrations of 3, 4, and 5 mM (P < 0.05). However, the difference in viability percentages at 1- and 2-mM concentrations was not significant compared to the control group (P > 0.05).
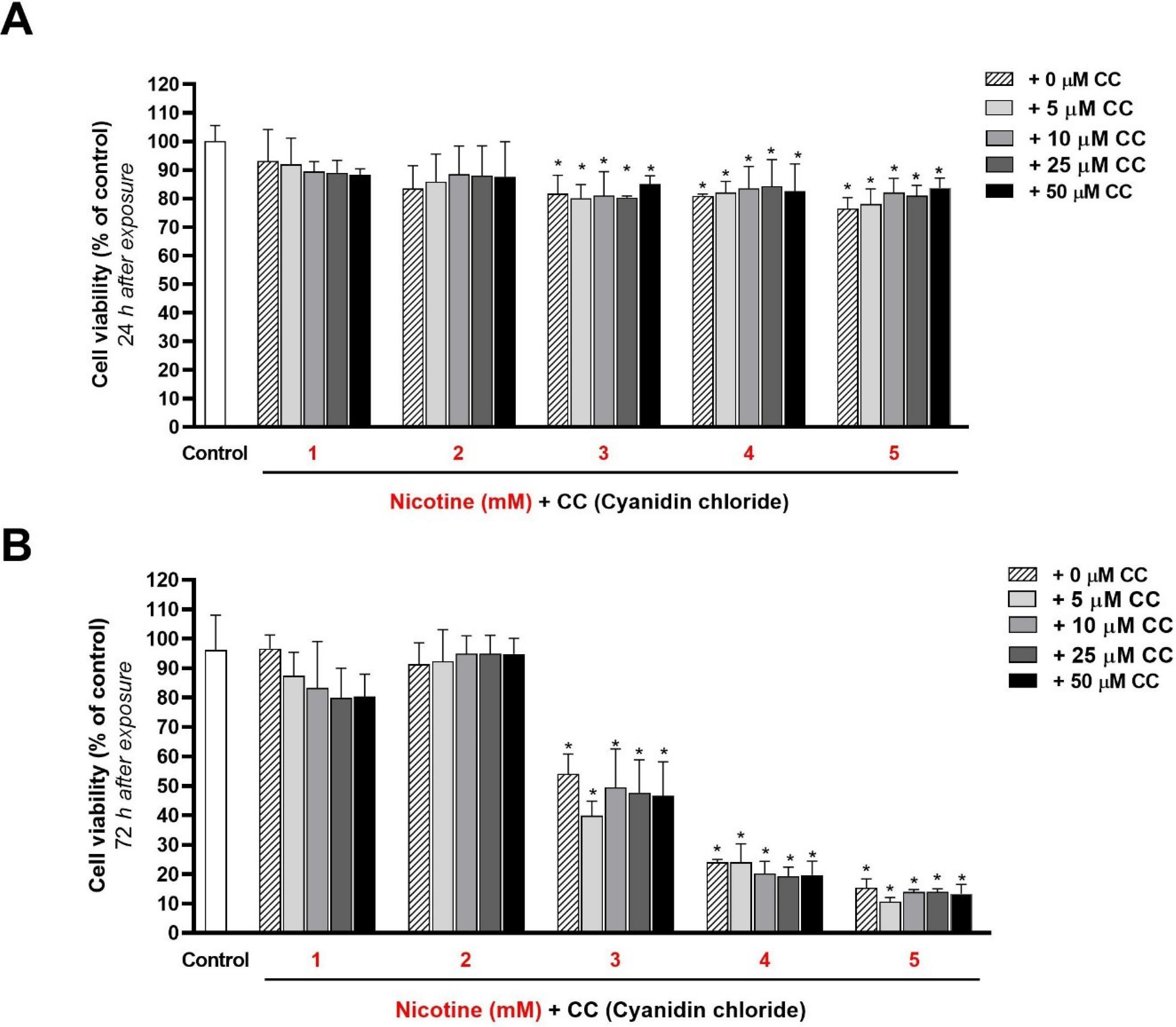
Figure 3.
Quantitative evaluation (MTT test) of the effect of different concentrations of nicotine (1 to 5 mM) on the viability and proliferation of HGFs in the presence and absence of different concentrations (0 to 50 μM) of anthocyanin cyanidin chloride (CC), 24 (A) and 72 (B) hours after exposure. The stars on the columns show a statistically significant difference in the survival percentage of the target group compared to the control group (100% viability - zero concentration of nicotine and cyanidin chloride) (P < 0.05)
.
Quantitative evaluation (MTT test) of the effect of different concentrations of nicotine (1 to 5 mM) on the viability and proliferation of HGFs in the presence and absence of different concentrations (0 to 50 μM) of anthocyanin cyanidin chloride (CC), 24 (A) and 72 (B) hours after exposure. The stars on the columns show a statistically significant difference in the survival percentage of the target group compared to the control group (100% viability - zero concentration of nicotine and cyanidin chloride) (P < 0.05)
According to the results, adding CC at concentrations of 5, 10, 25, and 50 μM could not significantly change (increase or decrease) cell viability percentages in the cells exposed to all five nicotine concentrations (P < 0.05).
After 72 hours of exposing HGFs to 1- and 2-mM concentrations of nicotine (Figure 3B) (from the results of the MTT quantitative test), no statistically significant increase or decrease in viability percentage was observed in the control group (P > 0.05). The results also showed that adding 5-, 10-, 25-, and 50-μM concentrations of CC to the cells stimulated with nicotine resulted in no statistically significant difference in cell viability.
A dose-dependent decrease in cell viability was found with 3-, 4-, and 5-mM concentrations of nicotine 72 hours after exposure (30‒90% reduction in viability percentage compared to the control group). Adding 5-, 10-, 25-, and 50-μM concentrations of CC could not significantly change the cell viability percentage and reduce the adverse effects of nicotine.
Qualitative evaluation of the effect of nicotine on the HGFs’ migration in the presence and absence of different anthocyanin CC concentrations
According to the results of MTT tests in the previous stages and primary cell migration tests, a 2.5-mM concentration of nicotine was selected for the final study of cell migration. Twenty-four hours after scratching and exposure to the studied materials, the wound in the control group (without nicotine and CC) was almost completely closed (Figure 4). The wound was not entirely closed after 24 hours at any of the three CC concentrations examined (without nicotine).
In the group exposed to 2.5 mM of nicotine (without CC), the wound (space between the two edges of the scratch) opened more than (wider) zero time, and cytotoxicity was observed. However, cytotoxicity and reduced proliferation were seen more frequently in the nicotine-alone group than in the groups containing CC (without significant differences between concentrations).
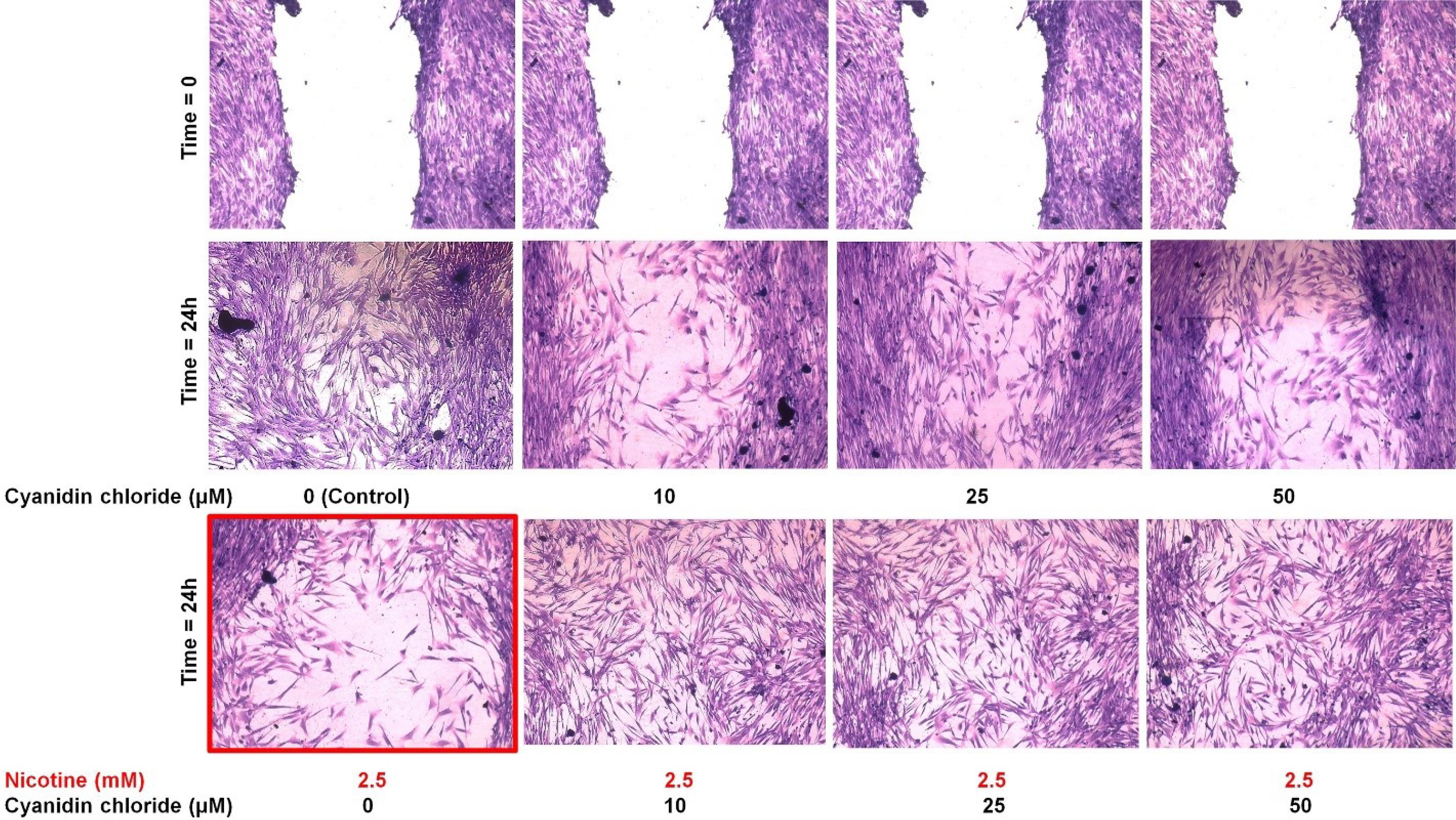
Figure 4.
Qualitative rate of migration of HGF cells alone (control: without nicotine and cyanidin chloride), in the presence of nicotine alone (2.5 mM), cyanidin chloride alone (10, 25, and 50 mM), and nicotine with cyanidin chloride in zero time (the moment of scratching and exposure to the studied materials) and 24 hours after exposure. In the group exposed to 2.5-mM nicotine (without CC), the wound (space between the two edges of the scratch) opened more than (wider) zero time, and cytotoxicity was observed (red borderline)
.
Qualitative rate of migration of HGF cells alone (control: without nicotine and cyanidin chloride), in the presence of nicotine alone (2.5 mM), cyanidin chloride alone (10, 25, and 50 mM), and nicotine with cyanidin chloride in zero time (the moment of scratching and exposure to the studied materials) and 24 hours after exposure. In the group exposed to 2.5-mM nicotine (without CC), the wound (space between the two edges of the scratch) opened more than (wider) zero time, and cytotoxicity was observed (red borderline)
Discussion
Today, we are witnessing an increase in smoking in different societies. Smoking is considered one of the most destructive risk factors for the development or progression of periodontal diseases. Tobacco smoke contains a variety of toxins, including nicotine (a highly addictive and fast-acting toxin), which affects many organs in the body. Cigarette nicotine significantly affects gingiva due to direct contact with epithelial cells and gingival fibroblasts. This substance prevents the adhesion and growth of gingival and periodontal ligament (PDL) fibroblasts.8,20,24-30
In 1998, Alpar et al31 examined the toxic effects of 0.48‒62-mM nicotine on two PDLs and HGF cell lines. The results showed that the cytotoxicity of nicotine in the studied cells was dose-dependent, and especially 6-mM (973 μg/mL), 8-mM (1298 μg/mL), and 10-mM (1620 μg/mL) concentrations of nicotine reduced the viability. This study also showed that nicotine at concentrations > 3.9 mM caused cytoplasmic vacuolization and changes in the cytoskeleton’s vital components, such as microtubules and actin filaments (which play an essential role in cell structure and mitosis). These morphological changes were reversible at concentrations of 1.9 to 10.3 mM (by removing nicotine from the culture medium), but the damage was irreversible at higher doses.
This in vitro study investigated the antioxidant potential of CC to reduce and antagonize the adverse biological effects of nicotine. According to the results of the cell viability and proliferation study by the MTT test, although the effect of this antioxidant in preventing the adverse biological effects of nicotine in the studied cells (HGF) was not significant, considerable effects were observed in the migration test and wound healing. However, due to the limitations of this laboratory study, confirmation of the initial cellular results requires more accurate cellular and molecular tests.
In the present study, 3‒5-mM concentrations of nicotine after 24 hours of exposure induced vacuolization of the cytoplasm and a dose- and time-dependent decrease in cell viability and proliferation. The results related to the adverse biological effects of nicotine are consistent with previous studies.31
This study showed that 2.5-mM nicotine reduced the density and migration rate of gingival fibroblasts, which can explain smokers’ slow wound healing rate. These results are consistent with studies that reported the adverse effects of smoking on wound healing. In 2005, a study showed that 16‒162 μg/mL of nicotine inhibited the HGF cell migration by acting on the Rac signaling pathway.21 In another study, HGFs were exposed to 0.025 to 32 μg/mL of nicotine, stimulating and inhibiting cell migration in low and high concentrations, respectively.10 Also, the results of a study in 2014 by Wheater and Mouabbi32 showed that nicotine at concentrations > 2 mM (400-400 μg/mL) was toxic to oral cells in a cell culture medium and reduced viability, adhesion, and migration of HGFs.
Since cigarette nicotine disrupts the balance of the cell oxidant (destructive free radicals)‒antioxidant (anti-free radical) system, it leads to the weakening and inefficiency of the antioxidant system (natural cell defense).14 Investigating the antioxidant effect is of great interest in antagonizing and reducing the adverse biological effects of nicotine. Studies have shown that the antioxidant activity of polyphenols in plants has many positive effects on human health. For example, polyphenols, including anthocyanins, can neutralize free radicals and thus protect the tissue against many chronic diseases. Anthocyanins (from the flavonoid family) are potent antioxidants hat neutralize free radicals due to their high reactivity.17
Few studies have investigated the antagonistic effect of antioxidants against nicotine.21,22,33,34 However, a comprehensive study on the antagonistic effect of the antioxidant anthocyanin CC against the toxic effects of nicotine has not been performed. In the present study, the toxic effects of different concentrations of nicotine, as well as the potential antagonistic effect of selected concentrations of CC on HGF cells, were investigated and compared.
In 2016, Torshabi et al21 conducted an in vitro study to investigate the effect of nicotine in the presence and absence of vitamin E, a potent antioxidant, on the morphology, viability, proliferation, and expression of osteogenic genes in MG-63 osteoblast-like cells. In 2017, they conducted an in vitro study examining nicotine and cotinine effects on MG-63 osteoblast-like cells and HGF gingival fibroblasts in the presence and absence of two potent antioxidants: vitamins C and E. The results of the above studies showed that vitamin E (alone or in combination with vitamin C) at a concentration of 5 mM was more effective than vitamin C (at a concentration of 1 mM) in antagonizing the adverse biological effects of nicotine and its metabolite namely cotinine (in 5 mM concentration) on the viability, proliferation, migration, differentiation, and apoptosis of the understudied cells.22
In 2010, San Miguel et al33 examined the effect of bioactive antioxidants (polyphenolic and turmeric derivatives at a concentration of 10−5 M of resveratrol (R), ferulic acid (F), phloretin (P), and tetrahydrocurcuminoids (T), [(RFT), (PFR), and (PF)]) on the proliferation and migration of human HGFs and periodontal ligament fibroblasts (HPDLs) pretreated with 6-mM nicotine for 2 hours. The results showed nicotine’s adverse biological effects on the viability and migration of the studied cells at concentrations > 2.5 mM, confirming the present study results. Also, they indicated that the cells treated with antioxidants showed more immigration rates than control cells and cells treated with nicotine. Double and especially triple combinations of these antioxidants (PFR and RFT) had more significant outcomes in counteracting nicotine effects and significantly increasing migration rates in HGF and HPDL than single antioxidants.35
In 2021, Damrongrungruang et al36 conducted an in vitro study to investigate the effect of anthocyanin complex (AC) composed of cyanidin-rich extract and delphinidin-rich extract, and AC noisome gel on oral wound healing, examining cell viability, cell migration, nuclear morphology, and protein expression of HGFs. The results showed a significant increase in cell viability after treatment with 0.002, 0.02, and 0.2 mg/mL of AC niosomes and 0.02, 0.2, and 2 mg/mL of AC. Moreover, the cell migration was significantly increased after 24 hours of treatment with AC and AC noisome gels.
In 2012, Desjardins et al35 showed that the anthocyanin cyanidin glucoside (at concentrations of 5 and 25 μg/mL) in combination with the other three anthocyanins (as black currant extract at concentrations of 5, 25, and 50 μg/mL) could significantly reduce the negative effect of nicotine on the viability of human gingival epithelial cells and fibroblasts at concentrations of 25 and 60 μg/mL of blackcurrant extract and 25 μg/mL of cyanidin glucoside. However, they did not examine cell migration.
In contrast to the present study, which used only one type of anthocyanin, the mentioned study used several anthocyanins with different concentrations; anthocyanins may have synergistic effects when combined. Therefore, it turns out that the biological effects of the anthocyanin CC, compared to other anthocyanins, and their synergistic effects should be further investigated in future studies.
In the present study, although different concentrations of CC alone (without nicotine) had no significant effect on the migration rate after 24 hours, significant effects were observed in improving migration (faster wound healing) in the presence of nicotine. As shown for the migration assay in Figure 4, nicotine alone adversely affected the migration of cells. The wound became worse and more open. However, the use of antioxidants led to healing of the wound and an increase in the number of cells.
These findings were compatible with a 2009 study by Nizamutdinova et al,37 which showed that a 50-μM concentration of anthocyanins in soy did not cause significant cell migration in dermal fibroblasts. On the other hand, in 2018, Hoskin et al38 reported that the polyphenols in blueberries could induce the migration of human dermal fibroblasts after 24 hours. Therefore, discrepancies in the effects of anthocyanins on cell migration might be attributed to differences in anthocyanin type and the studied cell type.
In 2011, San Miguel et al34 examined the effect of bioactive antioxidants (polyphenolic and turmeric derivatives at 10−3 to 10−5 M concentrations of resveratrol (R), ferulic acid (F), phloretin (P) and tetrahydrocurcuminoids (T); [(RFT), (PFR), and (PFT)]) on the proliferation and migration of HGFs and periodontal ligament fibroblasts (HPDLs). The results above showed that PFT increased cell migration; however, PFR and RFT did not significantly affect HGF wound healing rates.
The anti-inflammatory potential of these antioxidants might be more significant than other biological effects. For example, in 2012 and 2013, Tipton et al39,40 reported that proanthocyanidins, cyanidin groups, and peonidin groups of anthocyanins in cranberry extract might inhibit IL-6 and IL-8 production in normal HGFs. They also showed that these substances could modulate inflammatory and proteolytic processes in HGFs and aggressive periodontitis.
In contrast, a 2019 study by Jiang et al41 showed that CC (50 μM) could reduce the toxic effects of ZnO nanoparticles on colon cancer cells (CaCO2). However, this effect was not statistically significant. This substance could not significantly change the release rate of interleukin-8 induced by the above nanoparticles.
Conclusion
The present study showed that the adverse biological effects of nicotine on normal HGFs increased with increasing dose and time (dose-dependent and time-dependent toxicity). The antioxidants used in the present study could not reduce these adverse effects on cell viability and proliferation. However, anthocyanin and CC reduced the adverse effects of nicotine on cell migration to some extent. Due to the study’s limitations, additional comprehensive and cellular‒molecular research is necessary to evaluate this antioxidant’s beneficial biological effects. If the positive biological effects of the above antioxidant are proven, it can be used to prevent and treat oral diseases (gingival and periodontal tissues), especially in smokers.
Competing Interests
The authors state no conflict of interest.
Data Availability Statement
Data was presented in the manuscript.
Ethical Approval
The protocol of this study was approved by the Ethics Committee. (Ethical code: IR.SBMU.DRC.REC.1398.206).
Funding
The authors received no financial support for the publication of this article.
References
- Zulkefli N, Che Zahari CNM, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS. Flavonoids as potential wound-healing molecules: emphasis on pathways perspective. Int J Mol Sci 2023; 24(5):4607. doi: 10.3390/ijms24054607 [Crossref] [ Google Scholar]
- Buranasin P, Mizutani K, Iwasaki K, Pawaputanon Na Mahasarakham C, Kido D, Takeda K. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One 2018; 13(8):e0201855. doi: 10.1371/journal.pone.0201855 [Crossref] [ Google Scholar]
- Tatsumi M, Yanagita M, Yamashita M, Hasegawa S, Ikegami K, Kitamura M. Long-term exposure to cigarette smoke influences characteristics in human gingival fibroblasts. J Periodontal Res 2021; 56(5):951-63. doi: 10.1111/jre.12891 [Crossref] [ Google Scholar]
- Leite FRM, Nascimento GG, Scheutz F, López R. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med 2018; 54(6):831-41. doi: 10.1016/j.amepre.2018.02.014 [Crossref] [ Google Scholar]
- Holliday RS, Campbell J, Preshaw PM. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells A systematic review of the literature. J Dent 2019; 86:81-8. doi: 10.1016/j.jdent.2019.05.030 [Crossref] [ Google Scholar]
- Finklea JF, Hasselblad V, Riggan WB, Nelson WC, Hammer DI, Newill VA. Cigarette smoking and hemagglutination inhibition response to influenza after natural disease and immunization. Am Rev Respir Dis 1971; 104(3):368-76. doi: 10.1164/arrd.1971.104.3.368 [Crossref] [ Google Scholar]
- MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol 1992; 63(11):908-13. doi: 10.1902/jop.1992.63.11.908 [Crossref] [ Google Scholar]
- Chang YC, Huang FM, Tai KW, Yang LC, Chou MY. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodontal Res 2002; 37(4):279-85. doi: 10.1034/j.1600-0765.2002.01612.x [Crossref] [ Google Scholar]
- Giannopoulou C, Roehrich N, Mombelli A. Effect of nicotine-treated epithelial cells on the proliferation and collagen production of gingival fibroblasts. J Clin Periodontol 2001; 28(8):769-75. doi: 10.1034/j.1600-051x.2001.280808.x [Crossref] [ Google Scholar]
- Silva D, Cáceres M, Arancibia R, Martínez C, Martínez J, Smith PC. Effects of cigarette smoke and nicotine on cell viability, migration and myofibroblastic differentiation. J Periodontal Res 2012; 47(5):599-607. doi: 10.1111/j.1600-0765.2012.01472.x [Crossref] [ Google Scholar]
- Sree SL, Mythili R. Antioxidants in periodontal diseases: a review. Indian J Multidiscip Dent 2011; 1(3):140-6. [ Google Scholar]
- Bawadi HA, Khader YS, Haroun TF, Al-Omari M, Tayyem RF. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. J Periodontal Res 2011; 46(1):74-81. doi: 10.1111/j.1600-0765.2010.01314.x [Crossref] [ Google Scholar]
- Seaman DR. Nutritional considerations in the treatment of soft tissue injuries. In: Functional Soft-Tissue Examination and Treatment by Manual Methods. 3rd ed. Boston: Jones & Bartlett Learning; 2007.
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients 2010; 2(11):1106-31. doi: 10.3390/nu2111106 [Crossref] [ Google Scholar]
- Chang YC, Lii CK, Tai KW, Chou MY. Adverse effects of arecoline and nicotine on human periodontal ligament fibroblasts in vitro. J Clin Periodontol 2001; 28(3):277-82. doi: 10.1034/j.1600-051x.2001.028003277.x [Crossref] [ Google Scholar]
- Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007; 43:160-232. doi: 10.1111/j.1600-0757.2006.00178.x [Crossref] [ Google Scholar]
- Galvano F, La Fauci L, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S. Cyanidins: metabolism and biological properties. J Nutr Biochem 2004; 15(1):2-11. doi: 10.1016/j.jnutbio.2003.07.004 [Crossref] [ Google Scholar]
- Albensi BC. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion?. Front Cell Dev Biol 2019; 7:154. doi: 10.3389/fcell.2019.00154 [Crossref] [ Google Scholar]
- Lee DY, Yun SM, Song MY, Jung K, Kim EH. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants (Basel) 2020; 9(4):285. doi: 10.3390/antiox9040285 [Crossref] [ Google Scholar]
- Rezaei Esfahrood Z, Zamanian A, Torshabi M, Abrishami M. The effect of nicotine and cotinine on human gingival fibroblasts attachment to root surfaces. J Basic Clin Physiol Pharmacol 2015; 26(5):517-22. doi: 10.1515/jbcpp-2014-0120 [Crossref] [ Google Scholar]
- Torshabi M, Rezaei Esfahrood Z, Gholamin P, Karami E. Effects of nicotine in the presence and absence of vitamin E on morphology, viability and osteogenic gene expression in MG-63 osteoblast-like cells. J Basic Clin Physiol Pharmacol 2016; 27(6):595-602. doi: 10.1515/jbcpp-2015-0143 [Crossref] [ Google Scholar]
- Torshabi M, Rezaei Esfahrood Z, Jamshidi M, Mansuri Torshizi A, Sotoudeh S. Efficacy of vitamins E and C for reversing the cytotoxic effects of nicotine and cotinine. Eur J Oral Sci 2017; 125(6):426-37. doi: 10.1111/eos.12375 [Crossref] [ Google Scholar]
- International Organization for Standardization (ISO). Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. Geneve, Switzerland: ISO;2009.
- Giannopoulou C, Geinoz A, Cimasoni G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J Clin Periodontol 1999; 26(1):49-55. doi: 10.1034/j.1600-051x.1999.260109.x [Crossref] [ Google Scholar]
- Green CR, Rodgman A. The Tobacco Chemists’ Research Conference: a half century forum for advances in analytical methodology of tobacco and its products. Recent Adv Tobacco Sci 1996; 22:131-304. [ Google Scholar]
- Jacob V, Vellappally S, Smejkalová J. The influence of cigarette smoking on various aspects of periodontal health. Acta Medica (Hradec Kralove) 2007; 50(1):3-5. [ Google Scholar]
- James JA, Sayers NM, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol 1999; 70(5):518-25. doi: 10.1902/jop.1999.70.5.518 [Crossref] [ Google Scholar]
- Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000 2007; 44:178-94. doi: 10.1111/j.1600-0757.2007.00212.x [Crossref] [ Google Scholar]
- Kinane DF, Peterson M, Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000 2006; 40:107-19. doi: 10.1111/j.1600-0757.2005.00136.x [Crossref] [ Google Scholar]
- Tanur E, McQuade MJ, McPherson JC, Al-Hashimi IH, Rivera-Hidalgo F. Effects of nicotine on the strength of attachment of gingival fibroblasts to glass and non-diseased human root surfaces. J Periodontol 2000; 71(5):717-22. doi: 10.1902/jop.2000.71.5.717 [Crossref] [ Google Scholar]
- Alpar B, Leyhausen G, Sapotnick A, Günay H, Geurtsen W. Nicotine-induced alterations in human primary periodontal ligament and gingiva fibroblast cultures. Clin Oral Investig 1998; 2(1):40-6. doi: 10.1007/s007840050042 [Crossref] [ Google Scholar]
- Wheater MA, Mouabbi A. Effects of cotinine on human gingival fibroblast migration. Dent Open J 2015; 2(1):25-31. [ Google Scholar]
- San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svoboda KK. Antioxidants counteract nicotine and promote migration via RacGTP in oral fibroblast cells. J Periodontol 2010; 81(11):1675-90. doi: 10.1902/jop.2010.100187 [Crossref] [ Google Scholar]
- San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svoboda KK. Bioactive antioxidant mixtures promote proliferation and migration on human oral fibroblasts. Arch Oral Biol 2011; 56(8):812-22. doi: 10.1016/j.archoralbio.2011.01.001 [Crossref] [ Google Scholar]
- Desjardins J, Tanabe S, Bergeron C, Gafner S, Grenier D. Anthocyanin-rich black currant extract and cyanidin-3-O-glucoside have cytoprotective and anti-inflammatory properties. J Med Food 2012; 15(12):1045-50. doi: 10.1089/jmf.2011.0316 [Crossref] [ Google Scholar]
- Damrongrungruang T, Paphangkorakit J, Limsitthichaikoon S, Khampaenjiraroch B, Davies MJ, Sungthong B. Anthocyanin complex niosome gel accelerates oral wound healing: in vitro and clinical studies. Nanomedicine 2021; 37:102423. doi: 10.1016/j.nano.2021.102423 [Crossref] [ Google Scholar]
- Nizamutdinova IT, Kim YM, Chung JI, Shin SC, Jeong YK, Seo HG. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem Toxicol 2009; 47(11):2806-12. doi: 10.1016/j.fct.2009.08.016 [Crossref] [ Google Scholar]
- Hoskin RT, Xiong J, Esposito DA, Lila MA. Blueberry polyphenol-protein food ingredients: the impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem 2019; 280:187-94. doi: 10.1016/j.foodchem.2018.12.046 [Crossref] [ Google Scholar]
- Tipton DA, Babu JP, Dabbous M. Effects of cranberry components on human aggressive periodontitis gingival fibroblasts. J Periodontal Res 2013; 48(4):433-42. doi: 10.1111/jre.12023 [Crossref] [ Google Scholar]
- Tipton DA, Cho S, Zacharia N, Dabbous MK. Inhibition of interleukin-17-stimulated interleukin-6 and -8 production by cranberry components in human gingival fibroblasts and epithelial cells. J Periodontal Res 2013; 48(5):638-46. doi: 10.1111/jre.12050 [Crossref] [ Google Scholar]
- Jiang L, Li Z, Xie Y, Liu L, Cao Y. Cyanidin chloride modestly protects Caco-2 cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem Toxicol 2019; 127:251-9. doi: 10.1016/j.fct.2019.03.047 [Crossref] [ Google Scholar]